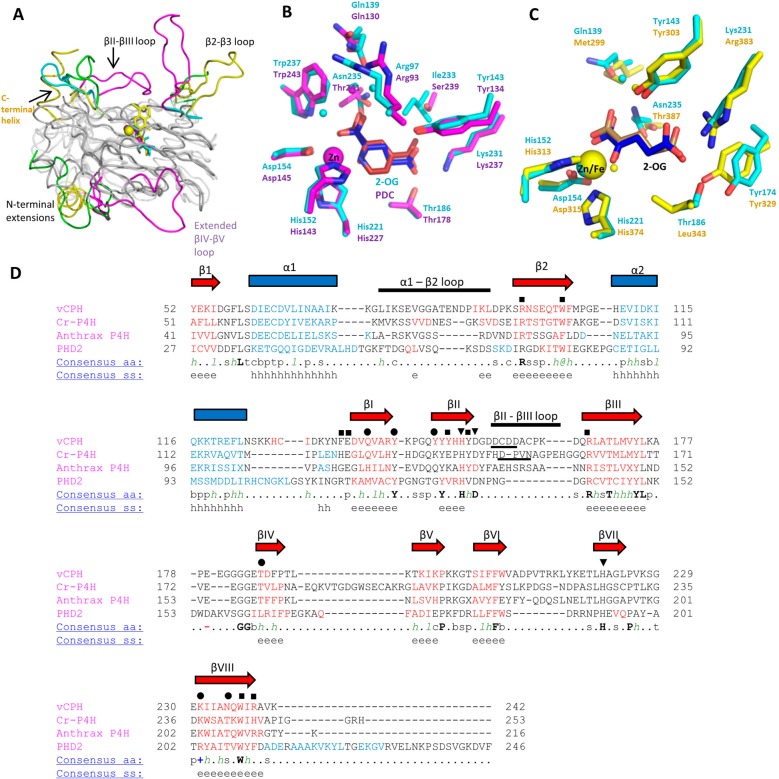
Figure 7.
Structural comparison of the prolyl-4-hydroxylases. (A) Superimposition of four available crystal structures of prolyl-4-hydroxylases. The core DSBH is colored gray, and the differing structural motifs are colored. vCPH is colored cyan, Cr-P4H magenta (PDB entry 2JIG), anthrax P4H green (PDB entry 3ITQ), and PHD2 yellow (PDB entry 2G19). Colored text refers to structures defined by the colors. (B) Comparison of vCPH (cyan) and Cr-P4H (magenta) (PDB entry 2JIG). Tyr149 from vCPH has been omitted for the sake of clarity. 2-OG from vCPH is colored blue and 2-OG from Cr-P4H red. (C) Comparison of vCPH (cyan) and PHD2 (yellow) (PDB entry 3OUJ). 2-OG from vCPH is colored blue and 2-OG from PHD2 brown. Water molecules are shown as small cyan, magenta, and yellow spheres for vCPH, Cr-P4H, and PHD2, respectively. (D) Structural alignment of the sequences of the prolyl-4-hydroxylases. Sequences were aligned by their secondary structures, using the Promal3D server. The secondary structure of vCPH is labeled above the sequence. β-Sheets are shown as red arrows and α-helices as blue squares. Residues involved in iron, 2-OG, and peptide binding are labeled as triangles, circles, and squares, respectively. Underlined residues in the βII−βIII loop indicate the conserved D/E-X-X-N/D motif. Key: α-helix, h; β-strand, e; conserved amino acids in bold and uppercase letters; aliphatic, l; aromatic, @; hydrophobic, h; alcohol, o; polar residues, p; tiny, t; small, s; bulky residues, b; positively charged, +; negatively charged, −; charged, c.