Figure 1.
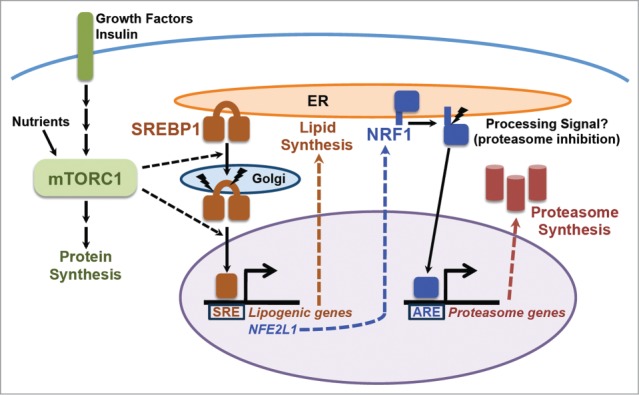
The mTORC1-SREBP-NRF1-Proteasome pathway. Growth factors and nutrients activate mTORC1, which promotes an increase in cellular protein synthesis. mTORC1 also stimulates activation of the SREBP1 transcription factor by promoting its processing and nuclear accumulation, which requires its trafficking to the Golgi, where it is proteolytically cleaved by 2 proteases, resulting in release of the N-terminus encompassing the mature active transcription factor. Mature SREBP1 binds to SRE sequences in the promoters of genes, including the enzymes of de novo lipid synthesis and NFE2L1, encoding the NRF1 transcription factor. NRF1 is synthesized as an ER transmembrane protein and must be processed to release the active transcription factor. Proteasome inhibitors stimulate this processing, but the nature of the physiological signal is currently unknown. Once activated, NRF1 goes to the nucleus and turns on a subset of genes containing AREs in their promoter, including those encoding all, or nearly all, subunits of the proteasome, leading to an increase in cellular proteasome content. See text for more details.
