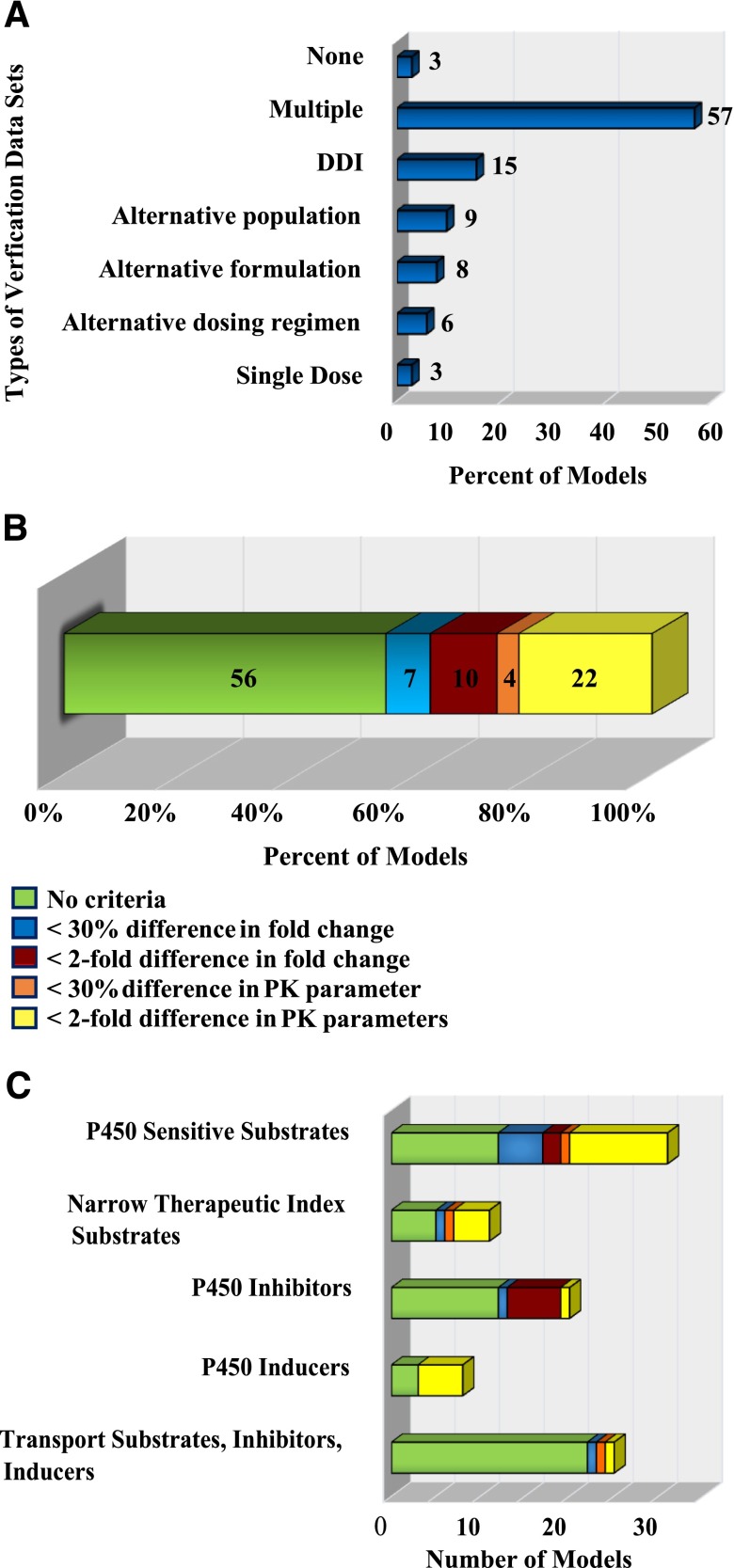
Fig. 2.
Summary of the verification criteria and alternative datasets used for the PBPK models in the literature evaluated. The types of in vivo datasets used to verify the quality of the models are shown in (A). The distribution of the acceptance criteria used in PBPK models of FDA probe substrates and inhibitors is shown in (B). The distribution of the model acceptance criteria used specifically for each compound class is shown in (C).