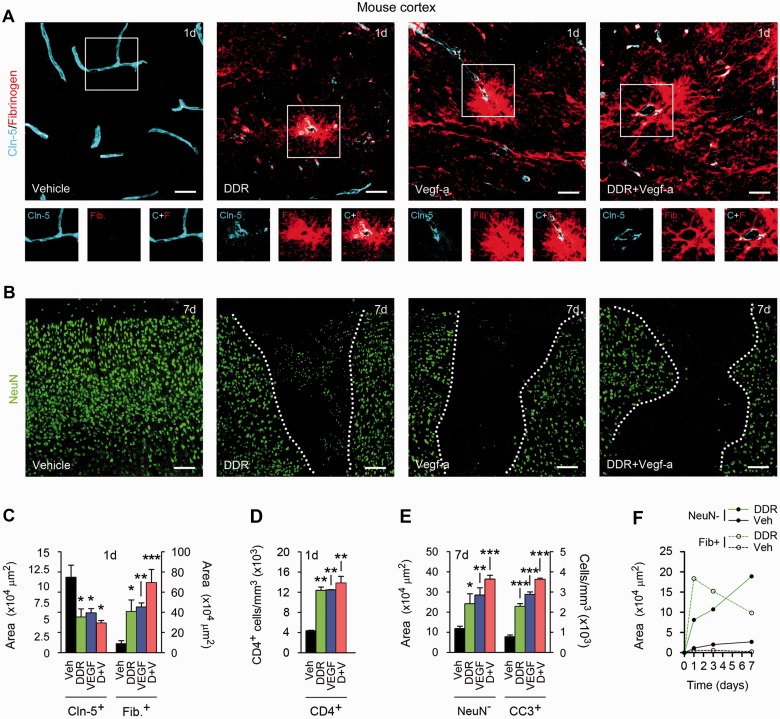
Figure 4.
DDR and VEGFA co-operate to produce blood–brain barrier breakdown in vivo. (A and B) Confocal Z-series projections of cerebral cortices of 12-week-old C57BL/6 mice 24 h (A) or 7 days (B) following stereotactic microinjection of murine VEGFA165 (60 ng in 3 µl PBS/BSA), and/or DDR (400 ng in 3 μl PBS), or vehicle control. Sections in (A) are immunostained for the endothelial tight junctions protein CLDN5 (blue channel) plus fibrinogen as a marker of blood–brain barrier breakdown (red channel). Both channels from areas outlined are shown below. Sections in B are stained for the neuronal marker NeuN (green channel). Morphometrically analysed data are presented in C–E. Blood–brain barrier disruption is seen around vessels in VEGF- or DDR-injected regions, and CLDN5 immuno-reactivity in these areas is reduced and discontinuous (A and C). These changes are associated with lymphocyte infiltration into VEGF- or DDR-injected locales (D). Blood–brain barrier breakdown is notably exacerbated in mice receiving VEGFA165 plus DDR together (A and C). Blood–brain barrier disruption in areas receiving VEGFA165 and/or DDR is followed by neuronal loss, and with the presence of apoptotic cleaved caspase-3+ NeuN+ neurons. These effects are also exacerbated in mice receiving VEGFA165 plus DDR in combination (B and E). (F) Time course morphometric measurements over a 7-day period post-injection reveals that blood–brain barrier disruption, as defined by the increase in area of parenchymal fibrinogen positivity, precedes neuronal loss (increase in area of NeuN− field) in DDR-induced lesions. Similar data are shown for VEGFA165-induced lesions in Supplementary Fig. 2A, which additionally demonstrates that co-treatment results in more persistent blood–brain barrier breakdown. Statistics: C–E = ANOVA plus Bonferroni post-test, *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars in A and B = 50 µm. Data shown are representative of findings from at least four mice per condition per time point.