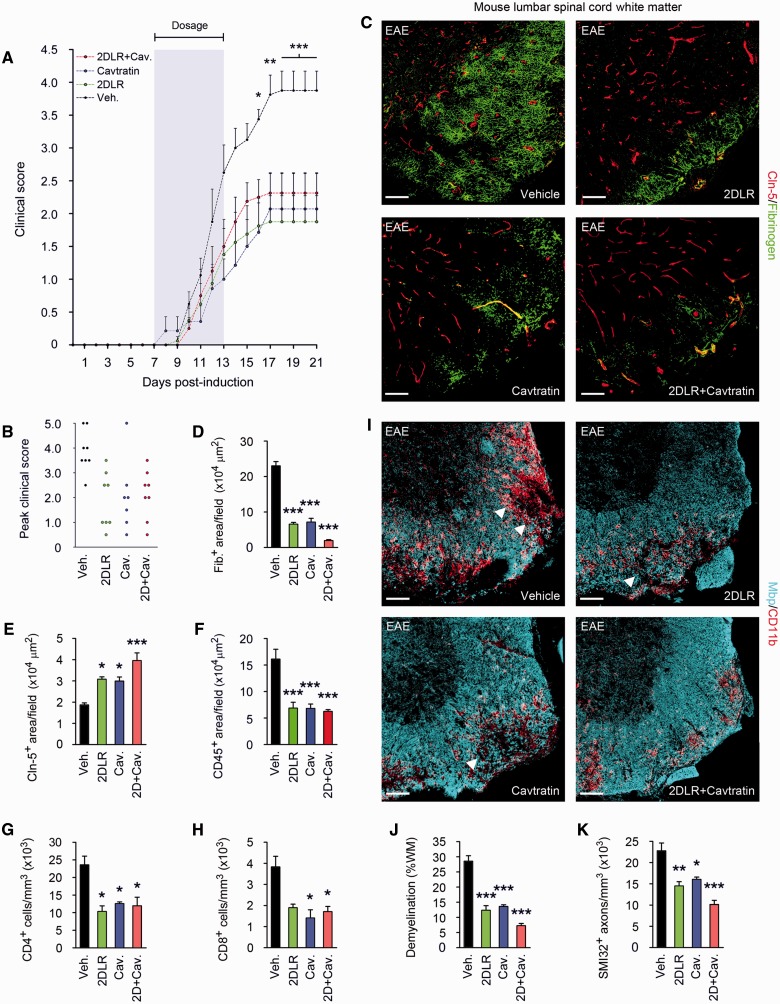
Figure 6.
Blockade of VEGFA and TYMP signalling reduces paralysis and tissue damage in EAE. (A) Mean clinical scores are shown from mice (8-week-old male C57BL/6, eight per group) sensitized with MOG35–55/CFA, then from onset of weight loss (7 days post-induction) treated for 7 days with 20 mg/kg/day 2DLR, and/or 2.5 mg/kg/day the inhibitor of VEGFA signalling, cavtratin, or vehicle control (shaded area). Neurological deficit has been scored daily for each animal according to a widely-used 5-point scale (EAE scoring: 1 limp tail; 2 limp tail and weakness of hind limb; 3 limp tail and complete paralysis of hind legs; 4 limp tail, complete hind leg and partial front leg paralysis) (Gurfein et al., 2009). Ascending paralysis is observed in all groups from 8 days post-induction, increasing in controls until signs stabilize at 17 days post-induction at a mean of 3.875, indicative of hindlimb paralysis plus mild forelimb weakness. Signs in 2DLR-treated or cavtratin-treated mice are much milder, stabilizing at 17 days at 1.875 or 2.07 (limp tail with hindlimb weakness), respectively. Interestingly, co-treatment does not produce additional clinical benefit. (B) Peak severity of paralysis is reduced in 2DLR-, cavtratin-, and co-treated mice. Controls (87.5%) but only 15–25% of 2DLR-, cavtratin- or co-treated mice display hindlimb paralysis or worse (score ≥3) during the disease course (21 days). Mortality or severe paralysis requiring euthanasia (score ≥4) reaches 50% in controls, but only 15% in the cavtratin-treated group, whereas mortality is absent in the 2DLR- and co-treated cohorts. (C–K) Neuropathological data from lumbar spinal cords of representative mice (three per group) from each cohort, sacrificed at 21 days. EAE controls display blood–brain barrier breakdown and disruption of endothelial CLDN5 (C–E), CD45+ inflammatory cell accumulation and CD4+ and CD8+ lymphocyte infiltration (F–H), demyelination and oligodendrocyte loss, and axonal transection (I–K). In I, representative areas of demyelination are arrowed. In 2DLR- and cavtratin-treated mice, CLDN5 expression is maintained and blood–brain barrier breakdown reduced (C–E), and inflammatory cell accumulation and lymphocyte infiltration restricted (F–H). Demyelination is decreased, and axonal integrity preserved (I–K). Importantly, although co-treatment is not associated with additional clinical benefits, it potentiates blood–brain barrier integrity and myelin and axon preservation (C–E and I–K). Scale bars in C and I = 50 µm. Statistics: A = two-way ANOVA plus Bonferroni test; D–H, J and K = one-way ANOVA plus Bonferroni test, *P < 0.05, **P < 0.01, ***P < 0.001. Data are representative of three independent experiments.