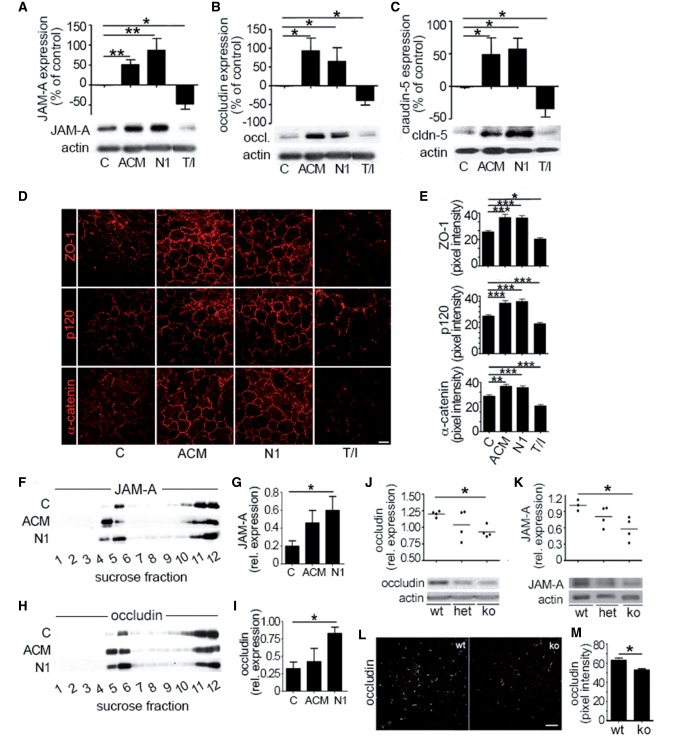
Figure 3.
Netrin 1 regulates junctional protein levels in vitro and in vivo. Expression of junctional proteins JAM-A (A), occludin (B), and claudin-5 (C) in primary cultures of human brain-derived endothelial cells untreated (control, C) or treated with astrocyte conditioned media, netrin I (N1) or T/I, by western blots. In vitro immunocytofluorescent stainings (D) of junctional proteins ZO-1, p120, and α-catenin in untreated (C), or in astrocyte conditioned media (ACM), netrin 1 or T/I treated brain-derived endothelial cells. Quantification of fluorescent pixel intensity of D is shown in E. Effect of netrin 1 on tight junction proteins JAM-A (F and G) and occludin (H and I) and accumulation into lipid raft membrane microdomains. Human brain-derived endothelial cells were treated as in A–C and cell membrane fractions were separated by sucrose density centrifugation. Lipid raft fractions (4 and 5) were isolated and western blot analysis of JAM-A (F and G) and occludin (H and I) was performed. Expression and quantification of tight junction proteins occludin (J), and JAM-A (K) in brain homogenates from netrin 1 knockout (ko) mice compared to heterozygote (het) and wild-type (wt) mice, by western blot. In situ immunostainings of occludin (L) in brains of netrin 1 knockout (ko) mice compared to wild-type mice. Quantification of fluorescence intensity of occludin expression is shown in (M). All data shown are the mean ± SEM from n ≥ 3–5 independent experiments, from three to five human brain-derived endothelial cell donors and/or ≥ 5 animals. Scale bars = 10 μm (D), 20 μm (L). (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).