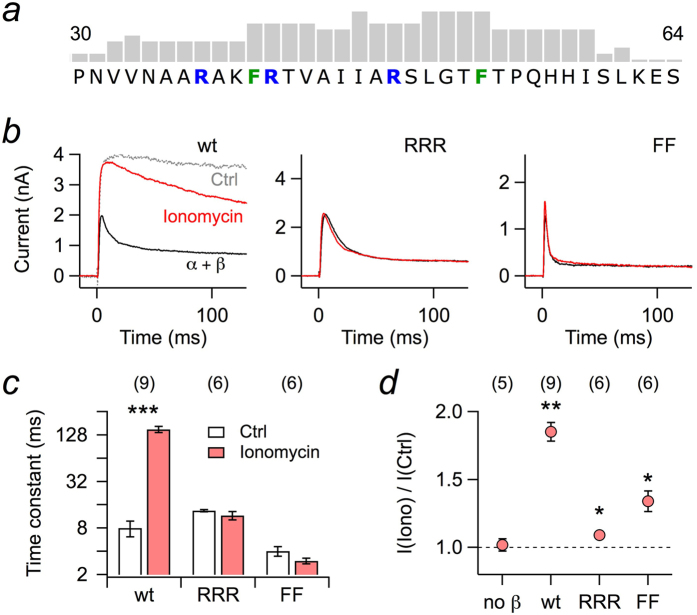
Figure 1. Whole-cell recordings of Kv1.1 currents from HEK 293T cells.
(a) Part of the N-terminal sequence of rat Kvβ1.1 protein with score pattern resulting from a search for potential calmodulin binding sites according to Mruk et al. (2014)25; the tallest bar refers to a score value of 9. Within this motif, either the marked arginine residues (RRR) or both phenylalanines (FF) were mutated to asparagine and serine, respectively. (b) Kv1.1 channels were coexpressed with Kvβ1.1 wild type (wt) or mutants RRR and FF in HEK 293T cells; currents were measured upon depolarization to 50 mV. The pipette solution contained 100 μM EGTA. Current traces for the indicated Kvβ1.1 subunits before (black) and after (red) extracellular application of 1 μM ionomycin. The grey trace in the left panel (Ctrl) indicates Kv1.1 currents without Kvβ subunits. (c) Inactivation time constants, based on single-exponential fits from data as shown in panel b. (d) Fractional change in peak current at 50 mV upon ionomycin application. Data in c and d are mean ± s.e.m. with n indicated in parentheses. Two-sided paired t-test between control and ionomycin application in c, Wilcoxon signed rank test in d: ***P < 0.001, **P < 0.01, *P < 0.05.