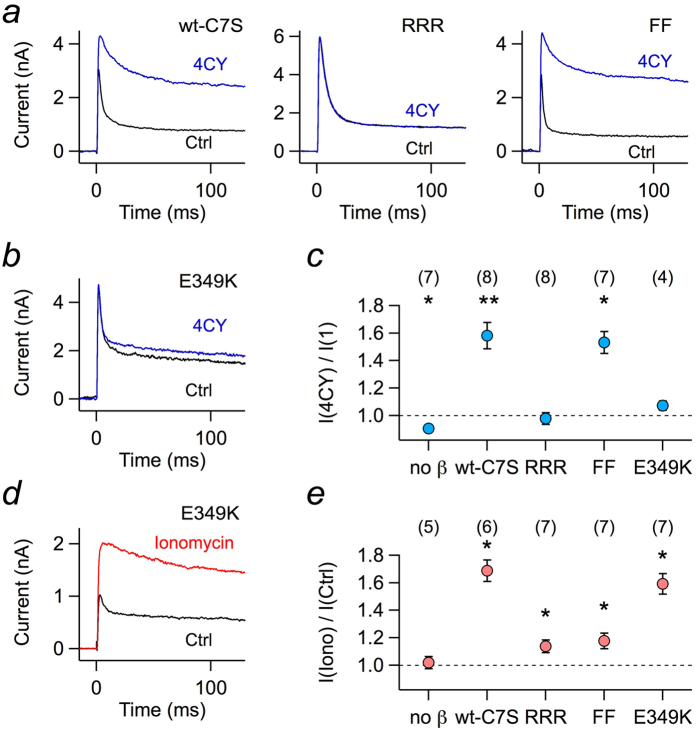
Figure 4. Enzymatic activity of Kvβ1.1-C7S variants.
(a) Superposition of current traces at 50 mV of Kv1.1 coexpressed in HEK 293T cells with Kvβ1.1-C7S (wt-C7S) or its mutants RRR and FF right after establishment of the whole-cell configuration (black) and about 150 s thereafter (blue). The pipette solution contained 1 mM of the substrate 4CY. (b) As in a, but with mutant Kvβ1.1-C7S-E349K. (c) Relative change in peak current from experiments as in a and b for Kv1.1 (no β) and coexpression with the indicated variants of Kvβ1.1-C7S. (d) Superposition of current traces at 50 mV for Kv1.1 plus Kvβ1.1-C7S-E349K before (black) and after extracellular application of 1 μM ionomycin (red). (e) Fold change of peak current upon ionomycin application for Kv1.1 (no β) and coexpression with the indicated variants of Kvβ1.1-C7S. The pipette solution contained 100 μM EGTA. Data in c and e are mean ± s.e.m. with n indicated in parentheses. Deviation from unity was tested with a Wilcoxon signed rank test: **P < 0.01, *P < 0.05.