Figure 5.
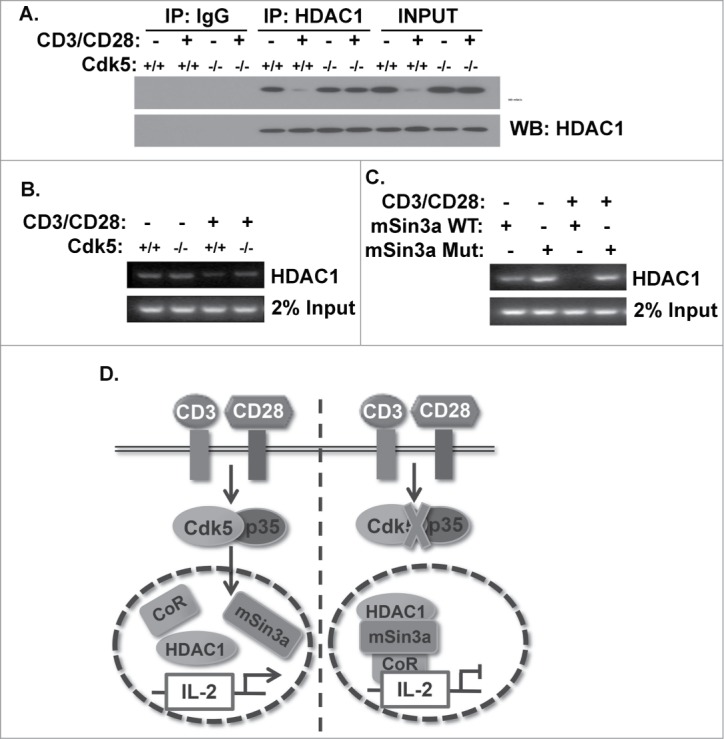
Phosphorylation of mSin3a by Cdk5 disrupts the formation of the HDAC1/mSin3a complex. (A) HDAC1 immunoprecipitates were isolated from nuclear lysates of primary wild type (Cdk5+/+) T cells or Cdk5−/- T cells either before or after stimulation with anti-CD3/CD28 antibodies. Immunoprecipitates were subsequently probed for mSin3a and HDAC1 expression by Western blot. (B) ChIP analysis was performed to assess the binding of HDAC1 to the IL-2 promoter in either Cdk5+/+ or Cdk5−/- T cells, either before or after activation with anti-CD3/CD28 stimulation. (C) Similarly, ChIP analyses were performed on Jurkat cells transfected with either Wild-Type (WT) or mutant (MUT) mSin3a plasmids to determine the binding of HDAC1 to the IL-2 promoter. (D) Diagram depicting the disruption of HDAC1 occupancy of the IL-2 promoter upon TCR stimulation, due to the presence of Cdk5/p35 activity, and the persistence of HDAC1 on the IL-2 promoter when the expression / activity of Cdk5 is disrupted.
