Abstract
Bees are model organisms for the study of learning and memory, yet nearly all such research to date has used a single reward, nectar. Many bees collect both nectar (carbohydrates) and pollen (protein) on a single foraging bout, sometimes from different plant species. We tested whether individual bumblebees could learn colour associations with nectar and pollen rewards simultaneously in a foraging scenario where one floral type offered only nectar and the other only pollen. We found that bees readily learned multiple reward–colour associations, and when presented with novel floral targets generalized to colours similar to those trained for each reward type. These results expand the ecological significance of work on bee learning and raise new questions regarding the cognitive ecology of pollination.
Keywords: Bombus, pollen, foraging, learning
1. Introduction
The complexity of ecological tasks means that animals must often learn not only conditional relationships with respect to a single reward type, but a multitude of relationships more or less simultaneously. While the ability to learn cues in different contexts is well studied (e.g. nest location or egg-laying versus foraging [1–3]), how multiple reward types are learned within a given context has rarely been addressed. Learning about different rewards is a critical skill for generalist foragers, for example when evaluating cues associated with alternative diets in relation to nutritional needs [3–6]. Foraging pollinators provide a unique opportunity to study multi-reward learning, because how effectively they learn about nutritionally complementary floral resources has important consequences not only for their own reproductive success, but also for that of the plant species they visit.
Over the past century, bees have emerged as a major system for the study of both the mechanisms and ecological consequences of learning. Generalist foragers collect nectar (their primary source of carbohydrates) and pollen (their primary source of protein) from a large variety of flowers and rapidly learn associations between floral colours and nectar [7]. However, bees can also learn visual associations with pollen [8,9], which many plants offer as their sole reward. Given that individuals of many species (including bumblebees [10]) collect both nectar and pollen during a single foraging bout, the question of whether bees can simultaneously learn floral cue associations with these two reward types is an important one, but surprisingly unaddressed. Ultimately, such insight might also be relevant for understanding why individuals vary in their tendency to collect multiple resources, if they are cognitively constrained [11].
We asked whether bumblebees Bombus impatiens could form associations between colour and both nectar and pollen when foraging simultaneously for these resources. We trained bees to visit artificial floral arrays where a target of one colour offered pollen and a target of another colour offered nectar. Because bees use different motor routines to collect nectar versus pollen, we were able to clearly identify which resource they attempted to collect. In an unrewarded test, we offered bees the two colours from training and two novel colours. If bees learned which flowers offered nectar and which offered pollen, we expected they would attempt to collect each reward from the colour previously associated with it. If bees learned which flower colour was unrewarding for a given reward type, we expected they would attempt to collect that reward from the novel colours in preference to the colour unrewarded during training.
2. Material and methods
We used three colonies (Koppert Biological Systems, MI, USA), represented equally across treatments. We connected these colonies one at a time to a foraging arena (L × W × H: 122 × 59 × 59 cm) for training and testing. The arena was lit by LED, fluorescent and natural light. We marked foragers that collected both nectar and pollen from a grey-flowered array with numbered thorax tags (Apinaut, Steißlingen, Germany; 39 bees).
We used flower-collected cherry pollen (Prunus avium; Firman Pollen, Yakima, WA, USA) and 50% (w/w) scented sucrose (100 ml water, 100 g sugar and 1 µl linalool) as a nectar surrogate throughout the experiment. We deprived colonies of pollen except for what bees collected during training and pipetted sucrose directly into honeypots daily (approx. two pots before training and approx. eight after).
(a). Protocol
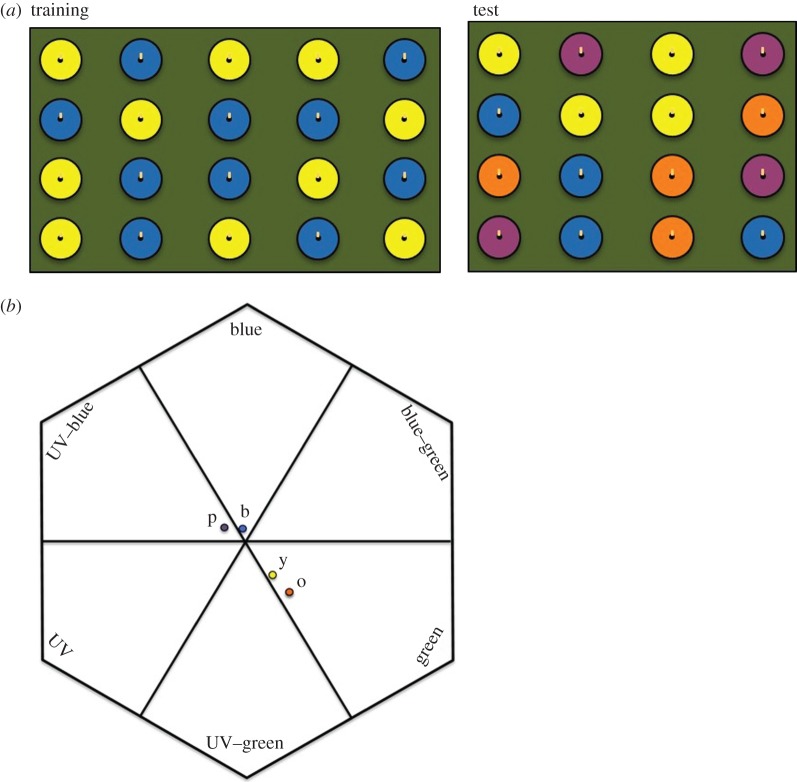
We trained bees on arrays of 20 artificial flowers (10 blue and 10 yellow; figure 1a; electronic supplementary material), employing a differential conditioning paradigm for each reward type: one colour of flower offered only pollen and the other only nectar (pollen – blue, nectar – yellow (PB-NY): n = 19; pollen – yellow, nectar – blue (PY-NB): n = 20). Nectar-rewarding flowers contained 12 µl of 50% scented sucrose in a well and an empty pollen-scented anther (details in the electronic supplementary material). Pollen-rewarding flowers contained approx. 3–5 mg of pollen on their artificial anther (chenille stem) and 12 µl of scented water in a well. On each of six training trials, we gave an individual access to the floral array to collect nectar and pollen before returning to the colony. Between each trial, we cleaned flowers with 70% ethanol, altered their positions on the array and replaced all nectar wells and anthers.
Figure 1.
(a) Training array with bee collecting pollen (left) and test array (right) (view the supplementary video to see nectar and pollen collection behaviour). (b) Flower colours plotted in bee colour space (y, yellow; o, orange; p, purple; b, blue; centre represents the background; relative distances in electronic supplementary material, table S1). (Online version in colour.)
Immediately after training, we presented subjects with a test array containing 16 unrewarding flowers ((human-) blue, yellow, orange and purple (figure 1a)) containing only scented anthers and scented water. These colours were chosen because they should be readily distinguishable to bees (figure 1b; see also the electronic supplementary material). After a bee had attempted 10 collections of each reward type, we removed her (usually less than 2 min).
We recorded trials from above using an HD Sony camcorder (30 fps) and coded behaviour frame-by-frame, noting whether each bee attempted to collect nectar (probed nectar well) or pollen (antenna/leg contact with anther) and whether she was correct (gained the reward) or incorrect (attempted to collect from an unrewarding flower) (electronic supplementary material and video). We carried out linear-mixed models (LMM) using the lme() function in the nlme package (R v. 2.15.1).
3. Results
(a). What did bees collect, and was this associated with performance?
During training, 20 bees (10 per treatment group) foraged for both reward types. Nineteen bees foraged mostly for only a single reward, in all cases after searching for the other reward type on the flower that was unrewarding for that reward type. These bees were significantly more likely to collect the reward type that was found on their preferred colour of flower (blue; [9]) (PB-NY: pollen-only: n = 6, nectar-only: n = 3; PY-NB: pollen-only: n = 2, nectar-only: n = 8; 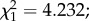 p < 0.05).
p < 0.05).
Among PY-NB bees, those that collected only nectar did not differ in training performance from bees that collected both rewards (F1,14 = 2.941, p = 0.108). However, among PB-NY bees, those that collected only pollen made fewer errors than bees that also collected nectar (F1,13 = 22.968, p < 0.0005). A similar relationship was found in the test phase results (full analyses and further details on single-reward foragers in the electronic supplementary material).
(b). Bees learned to associate colour with both reward types simultaneously
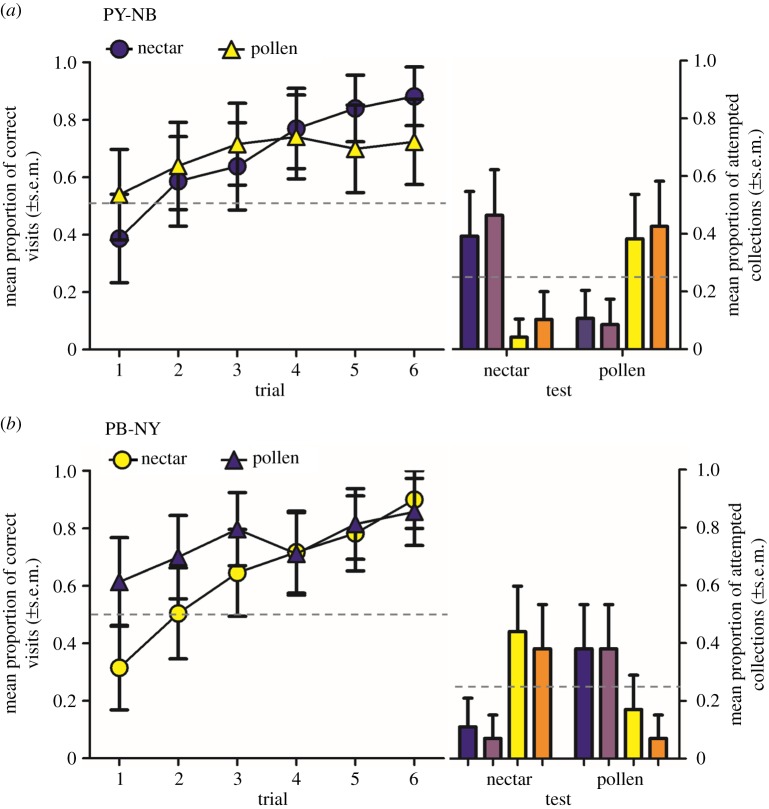
The bees that collected both rewards (N = 20) learned which flowers offered nectar versus pollen, making fewer errors in the colour of flower they visited for each reward across trials (LMM: F1,208 = 100.70; p < 0.0001; figure 2). Bees trained to PY-NB flowers learned at similar rates for each reward type (LMM of PY-NB treatment: reward type: F1,102 = 1.72; p = 0.19; trial: F1,102 = 38.85; p < 0.0001; figure 2a). Bees trained to PB-NY flowers made fewer errors when searching for pollen than nectar, but only in the first few trials (LMM of PB-NY treatment: reward type: F1,105 = 12.87; p < 0.001; trial: F1,105 = 64.49; p < 0.0001; reward type × trial: F1,105 = 8.98; p < 0.005; figure 2b).
Figure 2.
Learning and test performance for bees that foraged simultaneously for nectar (circles) and pollen (triangles) (n = 20), where (a) yellow flowers offer pollen, blue flowers nectar and (b) blue flowers offer pollen, yellow flowers nectar. Test data are the first 10 attempted collections of each reward type, averaged (±s.e.m.) across bees. Bar colour represents flower colour (left to right: blue, purple, yellow, orange). Dashed line indicates null expectation. (Online version in colour.)
In the test, bees that collected both nectar and pollen (n = 20) were more likely to search for a particular reward type on the colour that had previously offered that reward type (the CS+) than either the colour that was unrewarding for that reward type (the CS−) or the novel colour that was more similar to the CS− (t-values all > 3.0, p-values all <0.005; figure 2). However, bees were equally likely to search for a particular reward type on the CS+ as on the novel colour that was more similar to the CS+ (figure 1b; electronic supplementary material) (t-values all <1.5; p-values all >0.1).
4. Discussion
Although foragers require multiple nutritional resources, animal learning is most commonly studied in relation to a single reward. In unpredictable environments where food cues cannot be known in advance, associative learning is a process that could facilitate dietary mixing [4–6]. To date, evidence that foragers simultaneously show responses to multiple learned cues when collecting different nutrients has been lacking. Our findings support and extend earlier work showing that animals can learn to associate colour with artificial diets high or low in protein versus carbohydrates (e.g. [3,4]), by demonstrating that foragers not only learn these associations in an ecologically relevant scenario, but can do so when foraging simultaneously for both. Coping with multiple food types within the same context is likely to be a common feature of generalist foraging under natural circumstances (i.e. ‘food a but not food b is found in location x, while the reverse is true for location y′) and may present a cognitive challenge, but bees are clearly capable of meeting it.
Bees generalized across colour, treating purple flowers as if they were blue, and orange flowers as if they were yellow, even though the colour space model showed that they could likely readily distinguish these colours [12]. That bees generalize with pollen as they do for nectar [13] implies that individuals used the same ‘rules’ for generalizing for both reward types despite their collection requiring distinctly different motor routines. This generalization has implications for plant fitness, as it shows that bees form expectations about what type of reward a plant will offer based on its colour. Such an effect might promote convergence among co-flowering plant species that share both pollinators and concealed pollen rewards (cf. guild mimicry in [14]); alternatively, it might promote divergence among competitors offering nutritionally distinct resources (e.g. if pollen-motivated bees depress a similarly coloured nectar-rewarding plant's fitness [15]).
Further, we found that bees not only learned to collect one reward type on a given colour but also avoided collecting the other reward type on that colour. When an animal learns that a stimulus is rewarding for one reward type but not another, there is the potential for errors (e.g. by interference [1]). Our finding that bees in the PB-NY group that only collected pollen learned faster and performed better than bees that foraged for both nectar and pollen raises the possibility of a cognitive constraint shaping patterns of resource collection across a foraging bout [16] and/or a colony. However, because bees self-selected their level of specialization, this discovery requires further investigation to determine whether the difference truly reflects interference or is correlated with some other foraging-related trait.
The study of bee foraging behaviour has not only formed the basis of foundational work in behavioural ecology [17], but has also been described as a ‘magic well’ [18] of nearly limitless inspiration for researchers in cognitive neuroscience. Yet despite the degree of attention bees have received for their impressive cognitive abilities and a renewed emphasis on understanding how behavioural processes may contribute to their recent declines [19], our understanding of their foraging behaviour is incomplete in key respects. Our finding that bumblebees can simultaneously learn floral cues associated with pollen and nectar rewards is a step forward in that regard and opens the door to more ecologically realistic paradigms for the study of bee learning.
Supplementary Material
Acknowledgements
We thank J. Francis for comments and Firman Pollen for the pollen. Thanks to Avery Russell for coming up with the anther. Damian Sowers' web application greatly helped with the coding of behavioural data. Harvinder Singh provided invaluable help with data collection.
Data accessibility
All data associated with this project are archived at Dryad: doi:10.5061/dryad.0c96p.
Authors' contributions
F.M., D.P. and A.L. designed the study, F.M. collected the data. All authors wrote the manuscript, approve the final version, and agree to be held accountable for all the content included therein.
Competing interests
We have no competing interests.
Funding
This work was funded by NSF (IOS-1257762) to A.S.L. and D.R.P.
References
- 1.Weiss MR, Papaj DR. 2003. Colour learning in two behavioural contexts: how much can a butterfly keep in mind? Anim. Behav. 65, 425–434. ( 10.1006/anbe.2003.2084) [DOI] [Google Scholar]
- 2.Colborn M, Ahmad-Annuar A, Fauria K, Collett TS. 1999. Contextual modulation of visuomotor associations in bumble-bees (Bombus terrestris). Proc. R. Soc. Lond. B 266, 2413–2418. ( 10.1098/rspb.1999.0940) [DOI] [Google Scholar]
- 3.Lewis WJ, Takasu K. 1990. Use of learned odours by a parasitic wasp in accordance with host and food needs. Nature 348, 635–636. ( 10.1038/348635a0) [DOI] [Google Scholar]
- 4.Pérez C, Ackroff K, Sclafani A. 1996. Carbohydrate- and protein-conditioned flavor preferences: effects of nutrient preloads. Physiol. Behav. 59, 467–474. ( 10.1016/0031-9384(95)02085-3) [DOI] [PubMed] [Google Scholar]
- 5.Raubenheimer D, Tucker D. 1997. Associative learning by locusts: pairing of visual cues with consumption of protein and carbohydrate. Anim. Behav. 54, 1449–1459. ( 10.1006/anbe.1997.0542) [DOI] [PubMed] [Google Scholar]
- 6.Dukas R, Bernays EA. 2000. Learning improves growth rate in grasshoppers. Proc. Natl Acad. Sci. USA 97, 2637–2640. ( 10.1073/pnas.050461497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard AS, Masek P. 2014. Multisensory integration of colors and scents: insights from bees and flowers. J. Comp. Physiol. A 200, 463–474. ( 10.1007/s00359-014-0904-4) [DOI] [PubMed] [Google Scholar]
- 8.Nicholls E, Hempel de Ibarra N. 2014. Bees associate colour cues with differences in pollen rewards. J. Exp. Biol. 217, 2783–2788. ( 10.1242/jeb.106120) [DOI] [PubMed] [Google Scholar]
- 9.Muth F, Papaj DR, Leonard A. In press Bees remember flowers for more than one reason: pollen mediates associative learning. Anim. Behav . [Google Scholar]
- 10.Goulson D. 2003. Bumblebees: their behaviour and ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Bernays EA. 2001. Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 46, 703–727. ( 10.1146/annurev.ento.46.1.703) [DOI] [PubMed] [Google Scholar]
- 12.Dyer AG, Chittka L. 2004. Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a case study. J. Comp. Physiol. A 190, 105–114. ( 10.1007/s00359-003-0475-2) [DOI] [PubMed] [Google Scholar]
- 13.Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 48, 36–43. ( 10.1007/s002650000213) [DOI] [Google Scholar]
- 14.Jersáková J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 81, 219–235. ( 10.1017/S1464793105006986) [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves A, Harder L, Johnson S. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev. 84, 259–276. ( 10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- 16.Chittka L, Thomson JD. 1997. Sensori-motor learning and its relevance for task specialization in bumble bees. Behav. Ecol. Sociobiol. 41, 385–398. ( 10.1007/s002650050400) [DOI] [Google Scholar]
- 17.Von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 18.Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193, 801–824. ( 10.1007/s00359-007-0235-9) [DOI] [PubMed] [Google Scholar]
- 19.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this project are archived at Dryad: doi:10.5061/dryad.0c96p.