Figure 7.
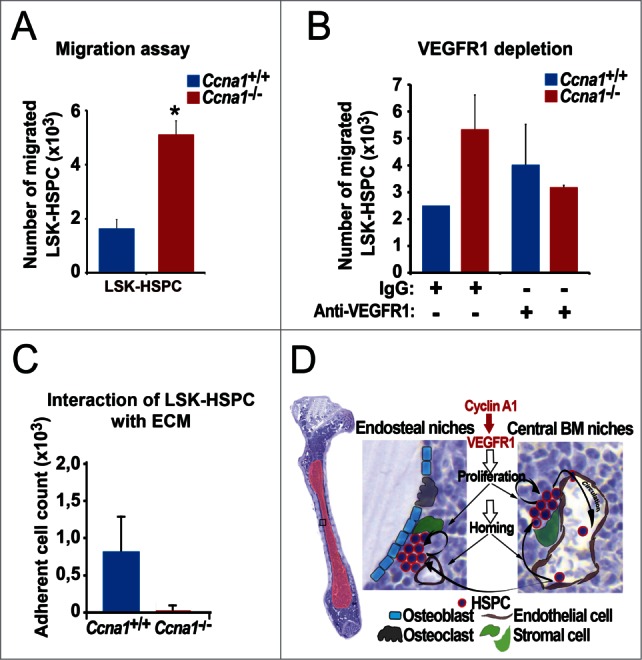
Altered VEGFR1 signaling in LSK-HSPC is in part responsible for altered migration and mobilization of LSK-HSPC in Ccna1−/− mice. (A) Migration assay to assess the migratory ability of sorted LSK-HSPCs cells from Ccna1−/− and Ccna1+/+ mice in in vitro is shown. Mean number of migrated Ccna1+/+ LSK-HSPC = 1621 per 10000 total cells applied in the migration assay; mean number of migrated Ccna1−/− LSK-HSPC = 5096, difference = 3475 cells, 95% CI = 1832 to 4288, p = 0.016. Data represent mean values + SEM from 3 independent experiments (n = 6 mice of each genotype). (B) Migration assay to evaluate the involvement of VEGFR1 in mediating the migratory ability of HSPCs from Ccna1−/ and Ccna1+/+ mice. For the blockage of VEGFR1 production, 1 µg/ml of anti-VEGFR1, or 1 µg/ml IgG antibody was used. p = 0.038. (C) Assessment of the interaction of LSK-HSPC from Ccna1−/ and Ccna1+/+ mice with fibronectin, an extracellular matrix component (ECM). The numbers of cells that are attached to the fibronectin-coated surface are shown. Data represent mean values + SEM from 2 independent experiments. (D) A schematic model to illustrate cyclin A1 function in the regulation of proliferation, homing and mobilization of HSPC and their interaction with the endothelial niche cells of perivascular vessels in the endosteal niches and central BM niches. Cyclin A1 regulates interaction of HSPC with their niches through VEGFR1. In both endosteal niches and central BM niches, endothelial niche cells of blood vessels that produce VEGFR1 may have a dominant and stimulatory influence on proliferation, migration and homing of HSPC under homeostatic conditions.
