Abstract
Introduction:
The administration of crystalloid fluids is considered as the first line treatment in management of trauma patients. Infusion of intravenous fluids leads to various changes in hemodynamic, metabolic and coagulation profiles of these patients. The present study attempted to survey some of these changes in patients with mild severity trauma following normal saline infusion.
Methods:
This study comprised 84 trauma patients with injury of mild severity in Shahid Rajaei Hospital, Shiraz, Iran, during 2010-2011. The coagulation and metabolic values of each patient were measured before and one and six hours after infusion of one liter normal saline. Then, the values of mentioned parameters on one and six hours after infusion were compared with baseline measures using repeated measures analysis of variance.
Results:
Eighty four patients included in the present study (76% male). Hemoglobin (Hb) (df: 2; F=32.7; p<0.001), hematocrit (Hct) (df: 2; F=30.7; p<0.001), white blood cells (WBC) (df: 2; F=10.6; p<0.001), and platelet count (df: 2; F=4.5; p=0.01) showed the decreasing pattern following infusion of one liter of normal saline. Coagulation markers were not affected during the time of study (p>0.05). The values of blood urea nitrogen (BUN) showed statistically significant decreasing pattern (df: 2; F=5.6; p=0.007). Pressure of carbon dioxide (PCO2) (df: 2; F=6.4; p=0.002), bicarbonate (HCO3) (df: 2; F=7.0; p=0.001), and base excess (BE) (df: 2; F=3.3; p=0.04) values showed a significant deteriorating changes following hydration therapy.
Conclusion:
It seems that, the infusion of one liter normal saline during one hour will cause a statistically significant decrease in Hb, Hct, WBC, platelet, BUN, BE, HCO3, and PCO2 in trauma patients with mild severity of injury and stable condition. The changes in, coagulation profiles, pH, PvO2, and electrolytes were not statistically remarkable.
Key Words: Fluid Therapy, blood gas analysis, hemodilution, multiple trauma
Introduction:
Trauma injury is one of the most important challenges confronting the field of medicine worldwide. Annually, almost five millions people die from injuries (1). Trauma, besides cancer and cardiac diseases are the leading causes of premature deaths in people before 65 years of age in many countries (2). Uncontrolled bleeding, followed by hemorrhagic shock and coagulation abnormalities, is the main cause of preventable death in these patients (3, 4). Fluid therapy is the cornerstone of treatment in such situation. The proper protocol of hydration therapy for trauma patients, has not yet been prepared. On the other hand, monitoring the hemodynamic and metabolic changes during resuscitation process is crucial (5-7). The effects of fluid therapy on hemodynamic and metabolic profile of the trauma patients are not completely clear. Only, there are few studies in this field which all are almost based on elective surgery patients, self-experiences, and experts' opinions (8-11). It seems that, the increased intravascular volume could have various effects on clinical and para-clinical aspects of patients. The increasing circulating volume leads to mounting the cardiac output by a heightened preload. The subsequent elevated cardiac output causes some changes in patient`s clinical findings such as blood pressure, heart rate, urine output, and skin temperature (12). There are several laboratory markers which are representative of tissue perfusion and metabolic changes during resuscitation (13, 14). These indices include base excess, serum lactate, tissue pH, and blood urea nitrogen (BUN) (14-16).The results of previous studies on effects of fluid therapy revealed various findings which could be due to the different situations, the severity of injury and initial hemodynamic and metabolic status of patients. To determine these effects, the present study was aimed to assess the changes in biochemical markers of trauma patients after infusion of one liter normal saline.
Methods:
The present study was conducted in trauma center of Shahid Rajaei hospital, Shiraz, Iran, in 2010-2011. The study protocol was approved by Ethics Committee of Shiraz University of Medical Sciences. Written informed consent was obtained from all patients. The patients younger than 16 and older than 60 years old, pregnant women, diabetic patients, those receiving blood transfusion, patients suffered from hepatic or cardiac failure, and subjects with coagulation abnormalities were excluded from the study. The severity of injury in all included patients was mild (score=4) based on revised trauma score (RTS). At the time of arrival to the emergency department, all the patients were visited and carefully examined by a general surgery resident. The metabolic and coagulation markers included complete blood count (CBC), BUN, Sodium (Na), Potassium (K), venous blood gas (VBG), international normalized ratio (INR), prothrombin time (PT), and partial thromboplastin time (PTT) were checked and entered to designed data form. Clinical values such as heart rate, blood pressure and respiratory rate were also measured and calculated. Then, one litter normal saline was infused to patients within one hour and the mentioned markers rechecked after one and six hours from admission time. All blood samples were derivate from the opposite site of punctured upper extremity.
Data were analyzed using the SPSS statistical software version 18.0. Quantitative data were expressed as mean ± standard deviation and qualitative ones as frequency and percentage. Repeated measures analysis of variances (ANOVA) was used to compare the clinical and biochemical values of patients at one and six hours after fluid therapy with base line. P value < 0.05 was considered significant.
Results:
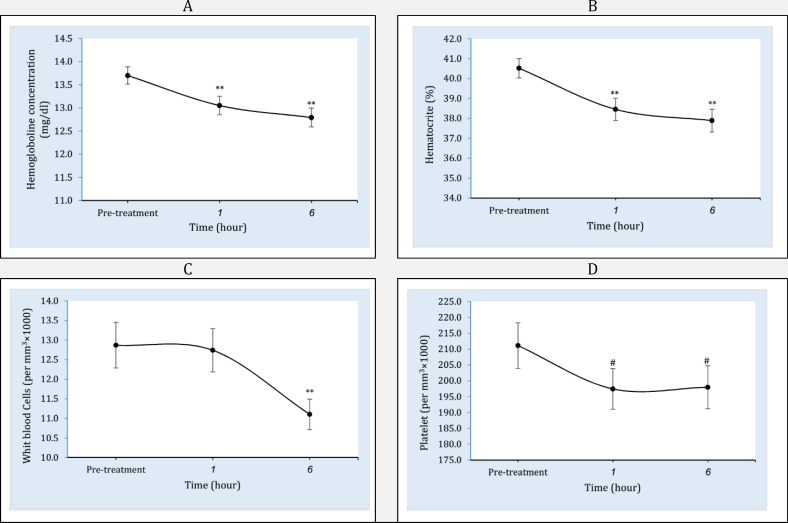
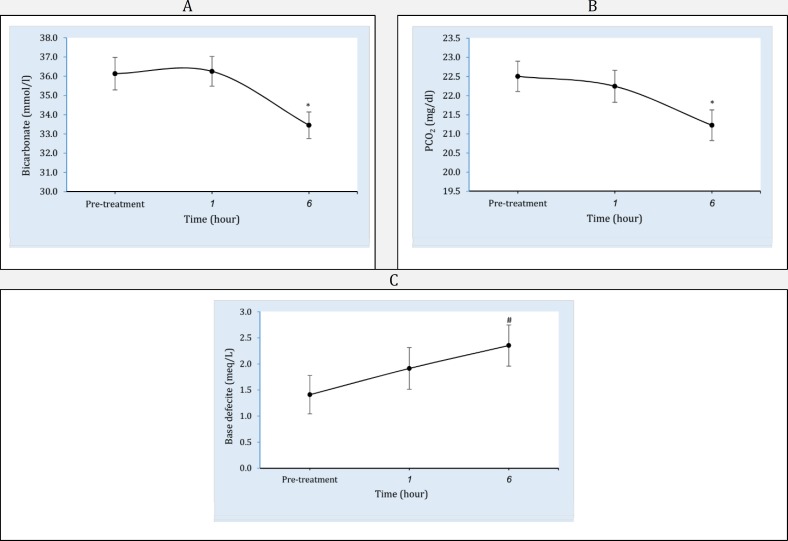
Of 84 patients who included in the present study, 64 (76%) were male. The mean age of patients was 32.1± 4.5 years. The types of injury were 54 (64.2%) long bones fracture, 9 (10.7%) head trauma, 4 (4.8%) chest trauma, and 17(20.2%) multiple trauma. The means of patients' systolic blood pressure, pulse rate, and respiratory rate on admission were 120.2±17.0 mmHg, 82.5±9.2/minute and 18.3±1.1/minute, respectively. Table 1 compares vital signs of patients among the time of admission, one hour, and six hours after infusion. Table 2 and figure 1 show the comparison of coagulation and metabolic factors on arrival time with those in one and six hours after infusion of one-liter normal saline. Hemoglobin (Hb) (df: 2; F=32.7; p<0.001) and hematocrit (Hct) (df: 2; F=30.7; p<0.001) levels both decreased one (pHb<0.001; pHct<0.001) and six (pHb<0.001; pHct<0.001) hours after infusion. Platelet count decreased after one hour (p<0.01) and fixed in six hours (p<0.35) (df: 2; F=4.5; p=0.01). White blood cells (WBC) decreased during the both studied times (df: 2; F=10.6; p<0.001). Coagulation markers were not affected during the time of study. The values of BUN showed statistically significant decreasing pattern following hydration therapy (df: 2; F=5.6; p=0.007). Table 3 and figure 2 shows the comparison of venous blood gas parameters on arrival time with those in one and six hours after infusion. Pressure of carbon dioxide (PCO2) (df: 2; F=6.4; p=0.002), bicarbonate (HCO3) (df: 2; F=7.0; p=0.001), and base excess (BE) (df: 2; F=3.3; p=0.04) values showed a significant deteriorating changes following infusion.
Table 1.
The comparison of vital signs on arrival time with those in one and six hours after infusion of one-liter normal saline (mean±SD)
| Vital signs | Admission | 1 hour | 6 hours | P |
|---|---|---|---|---|
| SBP (mmHg) | 120.2±17.0 | 120.9±12.8 | 120.0±11.0 | 0.91 |
| RR/minute | 18.3±1.1 | 18.9±2.3 | 18.7±2.3 | 0.42 |
| PR/minute | 82.5±9.2 | 81.2±7.8 | 84.2±9.5 | 0.74 |
SBP: systolic blood pressure; RR: respiratory rate; PR: pulse rate.
Table 2.
The comparison of coagulation and metabolic values (mean±SD) on arrival time with those in one and six hours after infusion of one-liter normal saline
| Characters | Admission | 1 hour | 6 hours | P |
|---|---|---|---|---|
| WBC/mm 3 | 12869.5±5272.5 | 12739.1±5012.8 | 11104.7±3492.0 | <0.001 |
| Hb (mg/dl) | 13.7±1.6 | 13.05±1.8 | 12.7±1.8 | <0.001 |
| Hct (%) | 40.5±4.4 | 38.4±5.09 | 37.9±5.1 | <0.001 |
| Plt/mm 3 | 211160±64812 | 197443±57081 | 197987±60765 | 0.01 |
| PT (second) | 13.04±1.8 | 13.6±1.8 | 12.9±1.6 | 0.21 |
| PTT (second) | 39.4±5.5 | 39.7±6.9 | 40.8±8.1 | 0.26 |
| INR | 1.15±0.2 | 1.15±0.2 | 1.15±0.2 | 0.67 |
| BUN (mg/dl) | 16.1±4.4 | 15.4±4.4 | 15.3±4.7 | 0.007 |
| Na (meq/l) | 141.7±3.8 | 141.2±4.1 | 140.6±4.7 | 0.048 |
| K (meq/l) | 4.0±0.5 | 4.0±0.4 | 4.1±0.5 | 0.32 |
WBC: White blood cell; Hb: hemoglobin; Hct: hematocrit; Plt: Platelet: PT: prothrombin time; PTT: partial thromboplastin time; INR: international normalized ratio; BUN: blood urea nitrogen; Na: Sodium; K: Potassium.
Figure 1.
Pattern of cell blood counts including hemoglobin (A), hematocrit (B), white blood cells (C), and platelet (D) changes following infusion of one liter normal saline at the time of study. ** Significant difference from pre-treatment time at level p<0.001. * Significant difference from baseline at level p<0.01. # Significant difference from baseline at level p<0.05
Table 3.
The comparison of venous blood gas parameters (VBG) on arrival time with those in one and six hours after infusion of one liter normal saline (mean±SD)
| parameters | Base line | 1 hour | 6 hours | P |
|---|---|---|---|---|
| P V O 2 (mmHg) | 42.6±26.45 | 45.5±19.1 | 45.3±19.8 | 0.38 |
| PCO 2 (mmHg) | 36.1±7.6 | 36.2±7.0 | 33.4±6.2 | 0.002 |
| HCO 3 (mmol/l) | 22.5±3.6 | 22.2±3.9 | 21.2±3.6 | 0.001 |
| BE (mEq/L) | -1.4±3.2 | -1.9±3.6 | -2.3±3.5 | 0.04 |
| pH | 7.41±0.07 | 7.40±0.05 | 7.42±0.05 | 0.08 |
VBG: venous blood gas; PO2: venous pressure of oxygen; PCO2: venous pressure of carbon dioxide; HCO3: Bicarbonate; BE: base excess.
Figure 2.
Pattern of venous blood gas parameters including bicarbonate (A), venous pressure of carbon dioxide (B), and base deficit (C) changes following infusion of one-liter normal saline at the time of study. ** Significant difference from baseline at level p<0.001. * Significant difference from baseline at level p<0.01. # Significant difference from pre-treatment time at level p<0.05
Discussion:
According to the findings of present study, although different metabolic and coagulation markers showed various patterns of changes, there were statistically significant changes only in Hb, Hct, platelet, and BUN values of patients following infusion of one liter normal saline. Also among VBG parameters PCO2, HCO3, and BE showed significant deteriorating pattern following hydration therapy.
Successful hydration therapy and fluid resuscitation is determined by maintenance of tissue perfusion. In this context, isotonic crystalloids usually are the first line fluid used in many trauma centers (17). On the other hand, crystalloids infusion leads to tissue edema, hemodilution and decreasing concentration of coagulative factors (18, 19). Despite positive effects of fluid infusion on vital signs, the reduction possible in oxygen-carrying capacity and consequently deteriorating tissue perfusion are undeniable side effects. In other word, the vital signs were maintained in patients by temporary increase in intravascular volume according to Frank Starling law, at the cost of decreasing oxygen-carrying capacity. The study of Lahsaee et al. showed significant decrease in Hb and Hct values following the hydration therapy in elective surgery patients (20). Crystalloid- induced hemodilution may be exacerbated with lower Hb in more severely-injured patients and unstable condition. Because of the importance of fluid therapy in management of trauma patients, removing it to conduct researches about the impact of fluid therapy on different indices is not ethical (19). But some studies conducted on animals, reported the effect of fluid therapy on indices such as blood pressure, lactate level, and Hct (19, 21). The effects of fluid therapy on BE and lactate have been studied in several investigations with different results (12, 14). Based on the finding of the present study, WBC count decreased one and six hour after normal saline infusion, which also may be due to hemodilution. The platelet count showed similar decreasing pattern to the WBC. Coagulation profile did not experienced any significant changes. This issue was against the findings of Chin LC et al., indicating the hypo-coagulation state as one of the important adverse effects of fluid therapy (22). There are few studies reporting sufficient amounts of VBG values in patients with injury of mild severity (23). In this study, the mean values of initial VBG were reported as near normal values in mild severity-injured trauma patients. Acidosis is considered as one of the hydration side effects, in Gerecht study (16, 24). But in the present study the pH level did not change significantly. This could be due the mild injury of the patients and low volume of fluid infusion. The finding of the present study revealed a worsening pattern of BE, PCO2, and HCO3 following infusion of one liter normal saline. It seems that monitoring and assessment of the changes in these values are necessary in patients with more severe injuries. Many studies have shown a reduction in BUN level as a result of the increasing of urine output after normal saline infusion in trauma patients. As well, in this study the BUN showed statistically significant decreasing changes after infusion of fluid. Further studies including patients with more severe injury are required to evaluate the impact of fluid therapy on different clinical and para-clinical aspects of trauma patients. Although more studies are needed in this area, it seems that we should revise our concept in trauma patients' resuscitation. However, it should be done noticing to patients` individual conditions, injury severity, vital signs, and primary laboratory findings such as Hb, BE, lactic acid and etc.
Conclusion:
It seems that, the infusion of one liter normal saline during one hour will cause a statistically significant decrease in Hb, Hct, WBC, platelet, BUN, BE, HCO3, and PCO2 in trauma patients with mild severity of injury and stable condition. The changes in coagulation profiles, pH, PvO2, and electrolytes were not statistically remarkable.
Acknowledgments:
The authors kindly appreciate the staffs of the trauma center of Shahid Rajaei hospital, Shiraz, Iran.
Conflict of interest:
None
Funding support:
None
Authors’ contributions:
All authors passed four criteria for authorship contribution based on recommendations of the International Committee of Medical Journal Editors.
References
- 1.World Health Organization, Violence Prevention Dept. The injury chart book: A graphical overview of the global burden of injuries: A graphical overview of the global burden of injuries. Genova: 2002. [Google Scholar]
- 2.Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death - United States, 2008-2010. Morb Mortal Wkly Rep. 2014;63(17):369–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Miniño AM. Statistics NCfH. Deaths: final data for 2004. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 4.Abbasi HR, Mousavi SM, Akerdi AT, Niakan MH, Bolandparvaz S, Paydar S. Pattern of Traumatic Injuries and Injury Severity Score in a Major Trauma Center in Shiraz, Southern Iran. Bull Emerg Trauma. 2013;1(2):81–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma Acute Care Surg. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. Journal of Trauma-Injury, Infection, and Critical Care. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB. Optimal use of blood products in severely injured trauma patients. Hematology Am Soc Hematol Educ Program. 2010;2010(1):465–9. doi: 10.1182/asheducation-2010.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tien H, Nascimento Jr B, Callum J, Rizoli S. An approach to transfusion and hemorrhage in trauma: current perspectives on restrictive transfusion strategies. Can J Surg. 2007;50(3) [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, Spinella PC. Optimal use of blood in trauma patients. Biologicals. 2010;38(1):72–7. doi: 10.1016/j.biologicals.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho AM-H, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? . Am J Surg. 2005;190(3):479–84. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury: incidence, risk stratification, and treatment options. Deutsches Ärzteblatt International. 2011;108(49):827. doi: 10.3238/arztebl.2011.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baratloo A, Rahmati F, Rouhipour A, et al. Correlation of Blood Gas Parameters with Central Venous Pressure in Patients with Septic Shock; a Pilot Study. Bull Emerg Trauma. 2014;2(2):77–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Kincaid EH, Miller PR, Meredith JW, Rahman N, Chang MC. Elevated arterial base deficit in trauma patients: a marker of impaired oxygen utilization. J Am Coll Surg. 1998;187(4):384–92. doi: 10.1016/s1072-7515(98)00202-6. [DOI] [PubMed] [Google Scholar]
- 15.Paladino L, Sinert R, Wallace D, Anderson T, Yadav K, Zehtabchi S. The utility of base deficit and arterial lactate in differentiating major from minor injury in trauma patients with normal vital signs. Resuscitation. 2008;77(3):363–8. doi: 10.1016/j.resuscitation.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Brunicardi F, Brandt M, Andersen D, et al. Schwartz's Principles of Surgery ABSITE and Board Review. McGraw Hill Professional; 2010. p. 60. [Google Scholar]
- 17.Moore KM. Controversies in fluid resuscitation. J Trauma Nurs. 2006;13(4):168–72. doi: 10.1097/00043860-200610000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Waikar SS, Chertow GM. Crystalloids versus colloids for resuscitation in shock. Curr Opin Nephrol Hypertens. 2000;9(5):501–4. doi: 10.1097/00041552-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lee C-C, Chang I, Yen Z-S, et al. Delayed fluid resuscitation in hemorrhagic shock induces proinflammatory cytokine response. Ann Emerg Med. 2007;49(1):37–44. doi: 10.1016/j.annemergmed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Lahsaee SM, Ghaffaripour S, Hejr H. The Effect of Routine Maintenance Intravenous Therapy on Hemoglobin Concentration and Hematocrit during Anesthesia in Adults. Bulletin of Emergency And Trauma. 2013;1(3 JUL):102–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y-Q, Cai X-J, Gu L-H, Wang Q, Huang W-D, Bao D-G. Experimental study of controlled fluid resuscitation in the treatment of severe and uncontrolled hemorrhagic shock. Journal of Trauma-Injury, Infection, and Critical Care. 2007;63(4):798–804. doi: 10.1097/TA.0b013e31815202c9. [DOI] [PubMed] [Google Scholar]
- 22.Chien L-C, Lu KJ, Wo CC, Shoemaker WC. Hemodynamic patterns preceding circulatory deterioration and death after trauma. J Trauma. 2007;62(4):928–32. doi: 10.1097/01.ta.0000215411.92950.72. [DOI] [PubMed] [Google Scholar]
- 23.Rudkin SE, Kahn CA, Oman JA, et al. Prospective correlation of arterial vs venous blood gas measurements in trauma patients. The American journal of emergency medicine. 2012;30(8):1371–7. doi: 10.1016/j.ajem.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerecht R. The lethal triad. Hypothermia, acidosis & coagulopathy create a deadly cycle for trauma patients. JEMS. 2014;39(4):56–60. [PubMed] [Google Scholar]