Abstract
Introduction:
Erythrocyte sedimentation rate (ESR) remains as one of the most reliable tests in clinical practices. Yet its use is time consuming and requires a large blood sample. The aim of this study was assessing a faster and reliable method of ESR estimation.
Methods:
An ESR estimation method was described and performed on 108 patients using capillary tube (micro ESR) and capillary peripheral blood. Micro ESR results at different intervals were measured and compared with Westergren ESR (conventional ESR) estimation by Pearson and Spearman’s coefficients. A regression equation was derived to predict conventional ESR values based on micro ESR results. The agreement of two measurements was demonstrated using the Bland-Altman plot.
Results:
Micro ESR results at 20 minutes showed the earliest close correlation with conventional ESR results at one hour (r = 0.987). The presented regression equation was able to closely predict ESR values (r2 = 0.974) and the Bland-Altman plot showed an acceptable agreement between converted and conventional ESR measurements.
Conclusion:
Using capillary tube and capillary blood sample (micro ESR) appears to be a faster, cheaper, more reliable, and precise tool for ESR measurement in the ED. The results have acceptable correlation with conventional ESR, especially at 20 minutes of measurement.
Key Words: Blood Sedimentation, methods, erythrocytes, capillary tubing, emergency medicine
Introduction:
Erythrocyte Sedimentation Rate (ESR) is one of the most commonly requested laboratory tests prescribed by physicians (1). Its rate is dependent on various physiologic and pathologic factors including hemoglobin concentration, ratio of plasma proteins, serum lipid concentration, and plasma pH (2). This limitation has not yet reduced the use of this test in different clinical settings. It is still often used to indicate disease severity and disease dynamic. In the emergency department (ED), ESR determination is an important part of critical and emergent diagnoses such as giant cell arteritis (GCA) and takes as an essential base for patient disposition in those with rheumatologic conditions (3). Furthermore, the role of ESR in clinical decision-making of non-emergent conditions has been reestablished in different settings including rheumatologic, hematologic, and even orthopedics (4-10).
Although Fahraeus and Westergren are often credited for the introduction of ESR and its clinical implications (11, 12), the test was originally described by Biernacki in Poland about a couple of decades before (13). It calculates the rate of sedimentation for red blood cells (RBC) within a 200 mm vertical tube of anticoagulated blood. Blood containing an anticoagulant remains as suspension for a relatively long time due to negative electrical charges on RBC surfaces (14).
The process of erythrocyte sedimentation is described in three phases: aggregation, precipitation, and packing; aggregation is the most influential phase in determining the outcome of the test (15). There are two main factors, which may influence the aggregation process: high molecular weight components of the plasma and RBC structure. Normally, RBCs have negative charges and repel each other; while, many plasma proteins have positive charges and neutralize the surface charges of erythrocytes, which promote the aggregation. Therefore, an increase in plasma proteins will be associated with higher ESR. The relative contribution of plasma proteins to aggregation on a scale of 10 is 10 for fibrinogen, five for beta-globulin, 2 for alpha-globulin, 2 for gamma-globulin, and 1 for albumin. On the other hand, ESR is directly proportionate to the mass of erythrocyte, but inversely related to the area of its surface. Macrocytes sediment more rapidly than normal cells and microcytes (14). The Westergren method for ESR estimation is simple and relatively cheap, but time-consuming and requires a relatively large volume of blood (15). Newer versions of ESR estimation have been proposed throughout the years and aimed to reduce its shortcomings while keeping its benefits (2, 16, 17). One of these flaws, especially in the emergency setting, is that the test requires at least 60 minutes, before it can be reported. An alternative method of ESR estimation, which has been first described by Stuart et al. (1974), is micro-ESR method, which uses capillary tube and capillary blood sample. It is observed that despite its shorter time requirement and no need for venipuncture, the results of this method are closely correlated with that of Westergren ESR. However, this method is not a routinely used in the ED. Therefore, the present study aimed to assess the validity of this old method for applying in emergency settings.
Methods:
Study design and setting
This was a cross-sectional study of patients’ required ESR analysis at Shohadaye Tajrish Hospital, Tehran, Iran. All patients requiring ESR evaluation, according to treating physician’s order, were included. In a one-month period, 108 patients were studied. All patients were asked to give an informed verbal consent. The study design was approved by the ethical committee of Shahid Beheshti University of Medical Sciences.
Procedure
For all participants ESR measuring was performed using both micro (Capillary tube) and conventional (Westergren) methods, simultaneously (11). Micro method was performed by the same researcher for all patients, while conventional method was conducted by laboratory technicians. The two sides were blinded to the result of the alternative technique. Since one of the study goals was finding the shortest time that has acceptable correlation with the findings of conventional method after 60 minutes, micro ESR results were documented at 10, 15, 20, 25, 30, 40, 50, and 60 minutes. In conventional method, at the time of sampling, 1.6 cc of patient’s whole blood was gently mixed with 0.4 cc of 3.8% sodium citrate. Then the anticoagulated blood was sucked into a glass Westergren pipette, placed into a stand, and fixed in vertical position for one hour. The sedimentation rate was estimated by measuring the column of serum at the top of the tube, based on millimeter per hour.
The required equipment for micro technique is listed in Table1. First, a drop of sodium citrate was put on a clean slide using a dropper. The patient’s fingertip was cleaned with an antiseptic agent; and punched using a lancet. The fingertip was held over the slide until four drops of blood were collected. Care was taken in not touching the slide with the finger so that full drops were collected and the 4:1 ratio between blood and sodium citrate was maintained. In case of failing to provide 4 drops, the finger was milked distally until the desired numbers of drops were collected. The collected blood was then gently mixed with the citrate on the slide using a plastic probe. Then a 7.5-centimeter capillary tube was placed on the slide immediately with a 30 to 45 degree angle. The tubes used, were heparin-free micro hematocrit tubes with an internal diameter of 1.2 millimeter (18, 19). The blood then rose into the tube due to capillary rise properties. If this fails to occur, the tube should be further lowered to smaller angle, while care should be taken to prevent entering air bobbles to the tube. After 7 centimeter rising in the tube, a finger was placed over the top end of the capillary tube to prevent further rise. The tube was then repositioned perpendicularly and placed into a standard micro-hematocrit seal paste in order to hold the tube in the desired vertical position. Burying 3 to 4 millimeter of the capillary bottom of the tube into the paste causes a further 1 to 2 millimeter rise in the column of blood. This was utter importance that the blood did not overflow. The tube should be fixed in a 90-degree angle to the surface, away from any vibrations. The level of blood was marked on the tube and then the rate of sedimentation within the tube documented at previously determined intervals, based on millimeter per hour.
Table 1.
Required equipment for capillary tube method of ESR measurement (micro ESR)
| Lancet |
| Sodium citrate |
| Dropper |
| Laboratory slide |
| Micro hematocrit capillary tubes |
| Antiseptic solution |
| Special rack with paste to hold capillary tubes in place |
| Ruler |
| Stopwatch |
Statistical analysis
The Data were analyzed using SPSS version 21.0 software. The correlation between micro ESR rates at different intervals (at 10, 15, 20, 25, 30, 40, 50, and 60 minutes) were compared with results of conventional method, as the gold standard, with Pearson and Spearman correlation method. A regression model was applied to calculate a formula for the conversion of the result of micro ESR values at the time of best correlation with conventional method. The micro ESR values were converted through this formula and then checked for having agreement with the gold standard using a Bland-Altman plot. In order to compare the variations between results, the coefficient of variation was calculated in both methods.
Results:
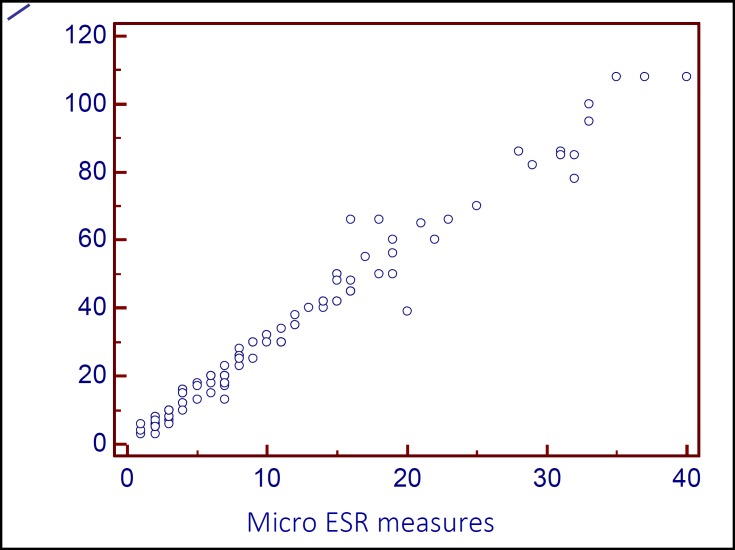
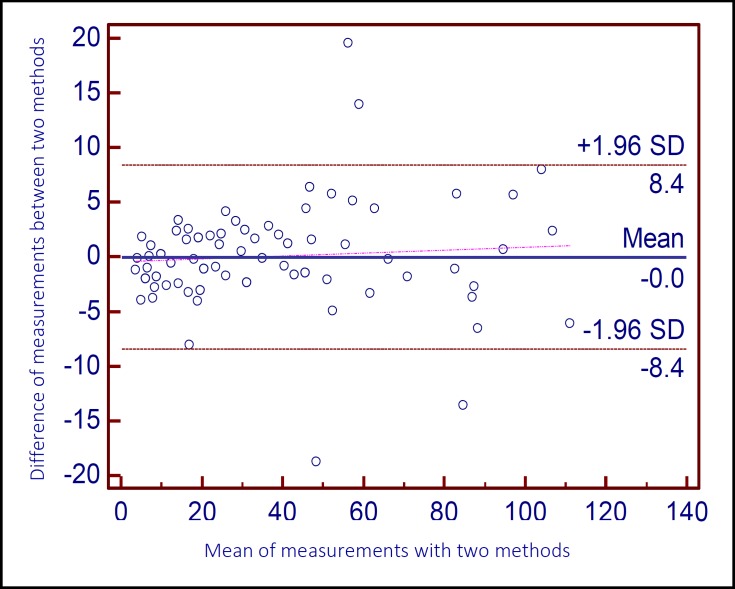
Overall, 108 patients were included (56.2% male). Patients had age range from 18 to 89 years. The earliest significant correlation was seen at 20 minutes with a remarkable correlation by Pearson’s method (r = 0.987, p < 0.001). Figure 1 shows the scatter plot of micro ESR values at 20 minutes and corresponding conventional ESR values. Since micro ESR and conventional ESR have different ranges of results, a regression equation was derived to predict conventional ESR values from micro ESR values at 20 minutes as follows: Conventional ESR values = 2.819 × micro ESR values at 20 minutes + 1.346 (r2 = 0.974). The Bland-Altman plot shows the agreement rate of converted results with conventional ESR values. This plot showed an acceptable agreement between the two values. It also showed better agreement at lower values of ESR (Figure 2). The coefficient of variation for conventional ESR and converted micro ESR results were 80% and 79%, respectively.
Figure 1.
Scatter plot of the result of micro ESR and conventional methods at 1 hour (p < 0.001). ESR: Erythrocyte sedimentation rate; CT-ESR 20 min: The value of ESR in 20 minutes in Capillary tube method ESR measurement (micro ESR
Figure 2.
Bland-Altman plot for assessment of agreement rate between the mean measurements of conventional ESR and converted micro ESR at different ESR values
Discussion:
The study results showed that micro ESR at 20 minutes had the earliest significant correlation with conventional ESR, which means that micro ESR results can be successfully interpreted after this time; thus, it can reduce the report time to a third of that in conventional ESR method. Furthermore, it was shown that accurate estimations of the conventional ESR could be made through correcting micro ESR measurements at 20 minutes by a simple formula presented here. This is helpful in situations where the clinician makes a decision based on previous measurements of recognized quantitative ESR. The correlation seen between the results of micro ESR and conventional ESR was proved excellent in this group of patients. ESR determination is one of the most basic tests performed in diagnostic laboratories. Its result has great importance for some critical diagnoses such as giant cell arteritis in the ED. It also takes as an important index in the management and disposition of patients with rheumatologic conditions as well as integral part of clinical decision-making for a wide range of medical conditions. However, the conventional method of Westergren, suggested by the International Committee for Standardization in Hematology as the standard procedure (20), is time consuming and requires a relatively large volume of patient’s blood. This is especially cumbersome for critically ill patients requiring multiple samplings, infants, and neonates. It has been suggested that removal of 1 milliliter of blood from a 1,000 gram infant is estimated to be equivalent to removing 70 milliliter from the average adult (16). Therefore, a faster but reliable test can greatly help in timely clinical decision making, while a simpler method of sample collection will be welcomed by both patients and clinicians. The conventional ESR method requires at least 1 hour of sedimentation. Here, we assessed a faster method of estimating ESR by means of capillary tubes and peripheral capillary blood. This was not the first time capillary blood and micro tubes used for the ESR estimation (18, 21-23); but, to our knowledge, this was the first time that such a small sample of capillary blood was applied. When the results were converted by the formula presented here, the results displayed a close agreement with that of conventional ESR. It seems that performing ESR using capillary tubes and capillary blood may be a reliable substitute for conventional ESR measurement. Furthermore, alongside its time saving and diagnostic accuracy, the method described here does not require venipuncture, requires less blood, less sophisticated equipment, and is more economical. All of these features in different parts such as screening, diagnosis, and follow up of the disease turn it to a more desirable and cost-effective alternative to the conventional method proposed by Westergren. Larger studies with wider range of patients will be required in the future to reaffirm the finding of this study.
Limitations
Although this study reveals a significant correlation between the proposed method and conventional ESR, its limitations should be taken into consideration. No strict inclusion or exclusion criteria were implemented here. In other words, no effort was made to gather abnormal results. Therefore, the majority of results were in normal limitations and fewer data in the extremes (where greater discrepancy might be expected) were available. Moreover, the new method of micro ESR is carried out only once on each patient and therefore the reproducibility of the results is not clear. Nevertheless, the impressive correlation and agreement of micro ESR with conventional ESR in a wide range of patients paves the way for assessment of its reproducibility and validity in extreme outcome of future studies.
Conclusion:
Using capillary tube and capillary blood sample (micro ESR) appears to be a faster, cheaper, more reliable, and precise tool for ESR measurement in the ED. The results have acceptable correlation with conventional ESR, especially at 20 minutes of measurement.
Acknowledgments:
We would like to acknowledge all the staff of Emergency ward and laboratory unit of Shohadaye Tajrish Hospital, Tehran, Iran.
Conflict of interest:
None
Funding support:
None
Authors’ contributions:
All authors met four recommended criteria of authorship released by international committee of medical journal editors (ICMJE). Reza Hashemi Contributed in the original research concept, study design and final composition of the manuscript. Alireza Majidi Contributed in the original research concept, study design and composition of the manuscript. Afshin Amini and Ali Tabatabaey Contributed in data collection, statistical analysis, and composition of the manuscript. Hassan Motamed Contributed in concept and data collection. Ali Tabatabaey and Afshin Amini wrote the first draft of the paper and others critically revised the manuscript. All authors approved of the final version of the manuscript and were accountable for all parts of the study.
References
- 1.Thue G, Sandberg S. Survey of office laboratory tests in general practice. Scand J Prim Health Care. 1994;12(2):77–83. doi: 10.3109/02813439409003679. [DOI] [PubMed] [Google Scholar]
- 2.Pawlotsky Y, Goasguen J, Guggenbuhl P, et al. Sigma ESR: an erythrocyte sedimentation rate adjusted for the hematocrit and hemoglobin concentration. Am J Clin Pathol. 2004;122(5):802–10. doi: 10.1309/8H4L-45F5-G0G1-VKY1. [DOI] [PubMed] [Google Scholar]
- 3.Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41(6):866–71. doi: 10.1016/j.semarthrit.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung R, Sillence DO, Tchan MC. Homocysteine and erythrocyte sedimentation rate correlate with cerebrovascular disease in fabry disease. JIMD Rep. 2012;6:101–5. doi: 10.1007/8904_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y, Park B, Kim K, et al. Erythrocyte sedimentation rate and anaemia are independent predictors of survival in patients with clear cell renal cell carcinoma. Br J Cancer. 2013;108(2):387–94. doi: 10.1038/bjc.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greidanus NV, Masri BA, Garbuz DS, et al. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am. 2007;89(7):1409–16. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- 7.Hauser G, Tkalcic M, Pletikosic S, Grabar N, Stimac D. Erythrocyte sedimentation rate - possible role in determining the existence of the low grade inflammation in Irritable Bowel Syndrome patients. Med Hypotheses. 2012;78(6):818–20. doi: 10.1016/j.mehy.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Stojan G, Fang H, Magder L, Petri M. Erythrocyte sedimentation rate is a predictor of renal and overall SLE disease activity. Lupus. 2013;22(8):827–34. doi: 10.1177/0961203313492578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamhane A, Redden DT, McGwin G Jr, et al. Comparison of the Disease Activity Score Using Erythrocyte Sedimentation Rate and C-reactive Protein in African Americans with Rheumatoid Arthritis. J Rheumatol. 2013;40(11):1812–22. doi: 10.3899/jrheum.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanem E, Antoci V, Jr, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444–9. doi: 10.1016/j.ijid.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Westergren A. The technique of the red cell sedimentation reaction. Am Rev Tuberc. 1926;14:94–101. [Google Scholar]
- 12.Westergren A. Diagnostic tests: the erythrocyte sedimentation rate range and limitations of the technique. Triangle. 1957;3(1):20–5. [PubMed] [Google Scholar]
- 13.Biernacki E. Samoistna sedymentacja krwi jako naukowa, praktyczno-kliniczna metoda badania. Gazeta Lekarska. 1897;17:962–96. [Google Scholar]
- 14.Sox HC Jr, Liang MH. The erythrocyte sedimentation rate. Guidelines for rational use. . Ann Intern Med. 1986;104(4):515–23. doi: 10.7326/0003-4819-104-4-515. [DOI] [PubMed] [Google Scholar]
- 15.Zhao TX, Lockner D. Electrical impedance and erythrocyte sedimentation rate (ESR) of blood. Biochim Biophys Acta. 1993;1153(2):243–8. doi: 10.1016/0005-2736(93)90411-r. [DOI] [PubMed] [Google Scholar]
- 16.Atas A, Cakmak A, Soran M, Karazeybek H. Comparative study between the Ves-matic and microerythrocyte sedimentation rate method. J Clin Lab Anal. 2008;22(1):70–2. doi: 10.1002/jcla.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull BS, Brailsford JD. The zeta sedimentation ratio. Blood. 1972;40(4):550–9. [PubMed] [Google Scholar]
- 18.Sabine JC, Nickolai DJ. A microhematocrit method and its use with citrated blood. Blood. 1952;7(11):1128–31. [PubMed] [Google Scholar]
- 19.Wongkrajang P, Opartkiattikul N, Chinswangwatanakul W, Areewatana S. Accuracy and precision evaluation of Thai plastic microhematocrit tubes: the first product from Thailand. J Med Assoc Thai. 2012;95(6):809–15. [PubMed] [Google Scholar]
- 20.ICSH recommendations for measurement of erythrocyte sedimentation rate. International Council for Standardization in Haematology (Expert Panel on Blood Rheology) J Clin Pathol. 1993;46(3):198–203. doi: 10.1136/jcp.46.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd HE. Estimation of the erythrocyte sedimentation rate of capillary blood; description of a new method. Ann Rheum Dis. 1958;17(2):234–9. doi: 10.1136/ard.17.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parida SN, Verma IC, Singh MB, Thomas S. Evaluation of micro erythrocyte sedimentation rate in the diagnosis of neonatal sepsis. Indian J Pediatr. 1980;47(388):381–4. doi: 10.1007/BF02759832. [DOI] [PubMed] [Google Scholar]
- 23.Douglas SE, Randolph TR. Development of a micro-ESR system with potential for in-home use. Clin Lab Sci. 2007;20(1):12–9. [PubMed] [Google Scholar]