Key Clinical Message
Sleeve gastrectomy (SG) is a surgical weight-loss procedure. Splenic abscess is a rare complication of SG. Four cases of splenic abscess after SG have been reported, all managed by surgical intervention. We report the first documented case of multiple splenic abscesses following SG managed conservatively by an integrated medical treatment.
Keywords: Conservative management, laparoscopic sleeve gastrectomy, morbid obesity, multiple splenic abscesses, postoperative complications
Introduction
Obesity is recognized as a global health crisis, and weight reduction has been demonstrated to improve survival and obesity-related comorbid conditions 1. As opposed to lifestyle modification and dietary programs, bariatric surgery has proved to be the most efficacious in the production of significant and durable weight loss 2. Nowadays, laparoscopic sleeve gastrectomy (LSG) is one of the most commonly performed bariatric surgical procedures in the management of morbid obesity 3.
There are many recognized complications of LSG 4. Early postoperative complications include bleeding, staple-line leak, and development of intraabdominal abscesses. Delayed complications include strictures, nutritional deficiencies, and gastroesophageal reflux disease 4. A splenic abscess, as a complication of SG, is extremely rare. To the best of our knowledge, only four cases have been reported to date in the medical literature and the management is always surgical 5–7.
We hereby present a case of multiple splenic abscesses secondary to LSG that was successfully managed conservatively by integrated medical treatment.
Case History
A 26-year-old male patient with a body mass index (BMI) of 44 kg/m2 (weight = 141 kg; height = 179 cm) underwent LSG on December 2013 to treat morbid obesity. During the operation, the liver, stomach, and spleen were of normal anatomy and there were no intraoperative complications. Postoperative course was uneventful. On day 7, a routine upper gastrointestinal gastrografin swallow study was performed and showed no evidence of leak or obstruction. Therefore, he was discharged home in a stable condition, followed a diet specifically devised for LSG recipients and was instructed to take daily vitamin supplements. To address a variety of topics including nutrition, exercise, and lifestyle changes, we normally offer free biweekly support to patients who undergo bariatric surgery. In this case, during the following weeks, the patient did not return for scheduled follow-up visits.
He remained well until 11 weeks later when he experienced persistent fevers (40.2°C), malaise, chills, and left upper quadrant abdominal pain. At this point, the patient had 30% loss of total weight (98.7 kg vs. 141 kg) and his BMI had reduced from 44 to 30.8 kg/m2. On postoperative day 77, he was readmitted to hospital.
Differential Diagnosis, Investigations, and Treatment
On physical examination, there was diffuse tenderness on the abdomen, especially on the left upper quadrant and spleen was enlarged 2 cm below the left costal margin. Laboratory tests revealed leucocytosis at 12.900/μL with 71.6% neutrophilia (neutrophil, 9.240/μL). Biochemical (albumin, total protein, transferrin, vitamin B12, folic acid, 25-vitamin D, vitamin B6, vitamin B1, calcium, and zinc) and immunological (absolute lymphocyte number) revealed malnutrition. Details are reported in Table1.
Table 1.
Patient's clinical characteristics at baseline and during the follow-up
| Clinical characteristics | Baseline (day 1) | After (day 7) | After (day 27) | After 2 months | Normal Values |
|---|---|---|---|---|---|
| Age (Years) | 26 | 26 | 26 | 27 | |
| Height (cm) | 179 | 179 | 179 | 179 | |
| Body Weight (kg) | 98.7 | 95.5 | 92.1 | 88.9 | |
| BMI (kg/m2) | 30.8 | 29.8 | 28.75 | 27.8 | |
| Fever (°C) | 40.2 | 36.5 | 36.1 | 36.4 | |
| Left upper quadrant tenderness | Present | Absent | Absent | Absent | |
| White blood cells (×103/μL) | 12.90 | 4.60 | 3.60 | 3.90 | 4.8–10.8 |
| Neutrophil (×103/μL) | 9.24 | 2.63 | 1.81 | 1.28 | 1.8–7 |
| Neutrophil (%) | 71.6 | 57.1 | 50.3 | 32.7 | 40–70 |
| Lymphocytes (×103/μL) | 0.92 | 1.30 | 1.34 | 2.24 | 1.0–4.8 |
| Lymphocytes (%) | 19.5 | 28.2 | 37.2 | 57.5 | 20–45 |
| Monocytes (×103/μL) | 0.98 | 0.55 | 0.35 | 0.31 | 0.1–0.8 |
| Monocytes (×103/μL) | 7.6 | 12.0 | 9.6 | 7.9 | 3–10 |
| Hemoglobin (g/dL) | 13.50 | 10.2 | 11.9 | 14.2 | 12–17.5 |
| Hematocrit (%) | 43.70 | 31.4 | 37.2 | 44.3 | 37–54 |
| Platelets (×103/μL) | 422 | 201 | 148 | 226 | 130–400 |
| Albumin (g/dL) | 2.8 | 3.6 | 5.4 | 4.5 | 3.6–5.2 |
| Total Protein (g/dL) | 7 | 6.6 | 8.0 | 6.9 | 6.5–8.2 |
| Transferrin (mg/dL) | 171 | 263 | 281 | 260 | 240–360 |
| Iron (mcg/dL) | 34 | 74 | 91 | 102 | 59–158 |
| Sodium (mmol/L) | 132 | 144 | 140 | 138 | 135–148 |
| Potassium (mmol/L) | 4.3 | 3.9 | 4.1 | 4.0 | 3.5–5.3 |
| Ferritin (ng/mL) | 461.2 | 291 | 201 | 223 | 30–400 |
| Vitamin B12 (pg/mL) | 113 | 151 | 234 | 451 | 157–1060 |
| Folic Acid (ng/mL) | 3.2 | 8.9 | 9.4 | 12.1 | 4.60–18.7 |
| Vitamin B1 (mg/L) | 1.6 | 1.84 | 1.96 | 2.05 | 1.50–2.30 |
| Vitamin B6 (mcgr/L) | 3.5 | 4.2 | 6.3 | 9.4 | 3.60–18 |
| 25-Vitamin D (ng/mL) | 4.9 | 5.1 | 6.1 | 8.3 | 20–30 |
| Calcium (mg/dL) | 8.5 | 9.2 | 9.4 | 9.3 | 8.6–10.3 |
| Zinc (mcg/dL) | 34 | 58 | 63 | 87 | 60–107 |
| C Reactive Protein (mg/dL) | 6.28 | 1.39 | 0.35 | 0.13 | 0.00–0.50 |
| Erythrocyte sedimentation rate (mm/h) | 57 | 20 | 4 | 4 | <15 |
| Fibrinogen (mg(dL) | 501.96 | 333.4 | 294.16 | 316.1 | 160–350 |
| Partial Thromboplastin Time [APTT] (sec) | 39.5 | 35.2 | 35 | 33.2 | 26–40 |
| Prothrombin time [PT] Activity (%) | 61.7 | 72.1 | 74 | 79.4 | 70–130 |
| PT/International Normalized ratio [INR] | 1.32 | 1.24 | 1.19 | 1.15 | 0.8–1.3 |
Hepatitis panel, toxicology, and serology for other potential viral and parasitic pathogens were negative. Echocardiogram found no evidence of bacterial endocarditis and upper gastrointestinal gastrografin swallow studies showed no evidence of leakage. The patient was immediately managed in a multidisciplinary way by radiologists, endoscopists, nutritionists, microbiologists, and surgeons. He remained hospitalized for 27 days.
On day 1, as shown in Figure1, abdominal computed tomography (CT) scan with oral contrast revealed three hypodence lesions within the splenic parenchyma, approximately 35 mm (a), 12 mm (b), and 0.86 mm (c) in diameter, respectively, compatible with multiple splenic abscesses, associated with mild splenomegaly. A partial percutaneous drainage of the larger abscess was then performed and the culture yielded Streptococcus anginosus. An antibiotic sensitivity test was performed using the disk diffusion and the agar dilution methods. The highest level of sensitivity, defined as minimum inhibitory concentration (MIC), was recorded for the fluoroquinolone drug class.
Figure 1.
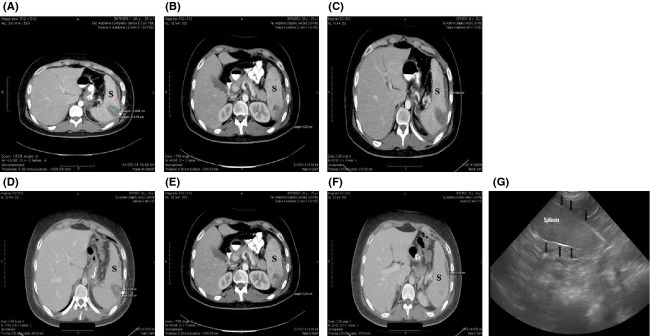
Axial contrast-enhanced CT images through upper abdomen show multiple hypodense lesions (A, B, C) in the spleen (S) consistent with splenic abscesses, associated with mild splenomegaly. A follow-up CT scan performed on the seventh day of admission showed a volumetric reduction of the three abscesses (D, E, F). A follow-up ultrasound images through upper abdomen (G) shows a normal splenic tissue. The tissue is homogeneous in texture and the margins of the capsule (black arrows) are smooth.
The patient was managed by an integrated medical therapy consisting of intravenous nutrition, fluid and electrolytes; intravenous culture-directed antibiotics; intravenous multivitamins, albumin, and proton pump inhibitors. In addition, anticoagulant medication was started with subcutaneous low-molecular-weight heparin. Details are reported in Table2.
Table 2.
Medical Integrated Therapy from day 1 to day 27
| Medicaments | Timing |
|---|---|
| Piperacillina (2 g) + Tazobactam (0.25 g) (Administered by intravenous infusion). | From day 1 to day 27, every 12 h |
| Levofloxacin 500 mg (Administered by intravenous infusion). | From day 1 to day 27, every 6 h |
| Albumin (Administered by intravenous infusion). | From day 1 to day 27, every 24 h |
| Parenteral Nutrition - PeriOLIMEL n° 4 (Administered by intravenous infusion). | From day 1 to day 27, every 24 h |
| Multivitamins - Cenervit (Administered by intravenous infusion). | From day 4 to day 27, every 24 h |
| Pantoprazole (40 mg) (Administered by intravenous infusion). | From day 1 to day 27, every 24 h |
Outcome and Follow-Up
A follow-up CT scan performed on the seventh day of admission (Fig.1b) showed a volumetric reduction in the three abscesses [28 vs. 35 mm (d); 1.0 vs. 12 mm (e), and 0.63 vs. 0.86 mm (f), respectively].
In addition, a methylene blue test was performed to further exclude staple-line leakage.
Also, as shown in Table1, improvement of his general condition was eminent on the seventh day of admission. Therefore, based on that together with some improvement of the abdominal symptoms and signs, the decision was made to continue the conservative management. He was discharged home in a stable condition on the 27th day (Table1).
At discharge, a daily oral proton pump inhibitor (Pantoprazole 40 mg) was prescribed for another month together with an oral antibiotic (Levofloxacin 500 mg, every 12 h) for a week. In addition, the patient was instructed to regularly follow a balanced diet 8 associated with daily vitamins and mineral supplements.
A follow-up ultrasound through upper abdomen two months later showed a complete resolution of the multiple splenic abscesses (Fig.1g) and the patient has had no subsequent recurrence of abdominal pain (Table1). Laboratory tests were normal (Table1). At his most recent follow-up visit, he had a total 14-month weight loss of 58 kg (BMI 25.9) and total weight loss of 41.2%.
Discussion
A splenic abscess as a complication of SG is extremely rare. To the best of our knowledge, there have only been four prior reports of splenic abscesses occurring after SG 5–7 and the management is always surgical. Predisposing conditions for splenic abscesses in patients that underwent SG 5 could include direct extension from a gastric staple-line leak, iatrogenic splenic injury at the time of the surgery, inadvertent splenic ischaemia after SG, and temporary immune suppression in the immediate postoperative period.
In this case, however, there was no gastric staple-line leak or iatrogenic splenic injury and/or ischaemia. Therefore, in this case, a bacterial translocation associated with the immuno suppression theory should be supposed. A partial percutaneous drainage of the larger abscess yielded S. anginosus, part of the human bacterial flora that rarely causes infections in healthy individuals but instead immunodeficient individuals are usually victim to this bacterium 9.
In conclusion, and in accordance with Sakran and coworkers 7, we theorized that temporary immuno suppression associated with rapid weight loss and limited oral intake, could possibly contribute to the formation of the multiple abscesses within the spleen from transient bacteria.
In fact, our patient followed this pattern. He was a young adult and developed multiple splenic abscesses 77 days after SG. Over 11 weeks, the patient had 30% total weight loss. Biochemical (albumin, total protein, transferrin, vitamin B12, folic acid, 25-vitamin D, vitamin B6, vitamin B1, calcium, and zinc) and immunological (absolute lymphocyte number) revealed malnutrition.
The common signs and symptoms of splenic abscess are nonspecific and include the triad of fever, left upper quadrant tenderness, and leucocytosis. In this case, the multiple abscesses became symptomatic in the intermediate period following surgery and were diagnosed with contrast CT scan.
In accordance with all reported studies, the CT scan remains the gold standard for definitive diagnosis 5–7, but ultrasound can also demonstrate the characteristics of splenic abscess. Both of these imaging studies have a sensitivity of 98% 10,11.
Classical teaching advocated splenectomy with antibiotics as it removes the complete focus of infection 12. However, recent evidence has shown increasing use of laparoscopic or percutaneous drainage of splenic abscess as an alternative, especially in a solitary splenic abscess. This is in contrast to multiple abscesses, which most commonly require splenectomy 12–15.
We report herein the first documented case of multiple splenic abscesses after LSG managed conservatively by an integrated medical treatment.
Conclusion
Splenic abscess is an extremely rare complication of SG. The management of multiple splenic abscesses after SG could be conservative in highly selected cases. The decision of adopting such conservative management should be based on the clinical condition of the patient and the response to initial treatment.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Conflict of Interest
The authors of this manuscript declare no conflict of interest regarding any commercial label or pharmaceutical industry.
References
- Bolen SD, Chang HY, Weiner JP, Richards TM, Shore AD, Goodwin SM, et al. Clinical outcomes after bariatric surgery: a five-year matched cohort analysis in seven US states. Obes. Surg. 2012;22:749–763. doi: 10.1007/s11695-012-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquitt J, Clegg A, Loveman E, Royle P. Sidhu MK. Surgery for morbid obesity. Cochrane Database Syst. Rev. 2005;4:CD003641. doi: 10.1002/14651858.CD003641.pub2. [DOI] [PubMed] [Google Scholar]
- Sherman V, Brethaer SA, Chand B. Schauer PR. Laparoscopic sleeve gastrectomy. In: Schauer PR, Schirmer BD, Brethaer SA, editors; Minimally invasive bariatric surgery. New York, NY: Springer Inc; 2007. pp. 173–178. in, eds., and. [Google Scholar]
- Sarkhosh K, Birch DW, Sharma A. Karmali S. Complications associated with laparoscopic sleeve gastrectomy for morbid obesity: a surgeon's guide. Can. J. Surg. 2013;56:347–352. doi: 10.1503/cjs.033511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y, Cawich S, Aziz I. Naraynsingh V. Delayed splenic abscess after laparoscopic sleeve gastrectomy. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-208057. , and doi: 10.1136/bcr-2014-208057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avulov V, McQuillen DP, Mizusawa M. Brams D. Splenic abscess after sleeve gastrectomy. SAGES Metab Obes Arch. 2014;7:71. [Google Scholar]
- Sakran N, Iliviitzki A, Zeina AR. Assalia A. Splenic abscess after sleeve gastrectomy: a report of two cases. Obes. Facts. 2012;5:635–639. doi: 10.1159/000342805. [DOI] [PubMed] [Google Scholar]
- Schiavo L, Scalera G, Sergio R, De Sena G, Pilone V. Barbarisi A. Clinical impact of Mediterranean-enriched-protein diet on liver size, visceral fat, fat mass, and fat free mass in patient undergoing sleeve gastrectomy. Surg. Obes. Relat. Dis. 2015 doi: 10.1016/j.soard.2015.04.003. , and in press. doi: http://dx.doi.org/10.1016/j.soard.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Ruoff KL. Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin. Microbiol. Rev. 1988;1:102–108. doi: 10.1128/cmr.1.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi LL, Nambiar R, Rauff A, Mack PO. Yap TL. Splenic Abscess. Aust. N. Z. J. Surg. 1992;62:780–784. doi: 10.1111/j.1445-2197.1992.tb06917.x. [DOI] [PubMed] [Google Scholar]
- Chang KC, Chuah SK, Changchien CS, Tsai TL, Lu SN, Chiu YC, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J. Gastroenterol. 2006;12:460–464. doi: 10.3748/wjg.v12.i3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell AM, Kercher KW, Matthews BD, Joels CS, Sing RF. Heniford BT. Laparoscopic splenectomy for splenic abscess. Surg. Laparosc. Endosc. Percutan. Tech. 2004;14:289–291. doi: 10.1097/00129689-200410000-00013. [DOI] [PubMed] [Google Scholar]
- Ng KK, Lee TY, Wan YL, Tan CF, Lui KW, Cheung YC, et al. Splenic abscess: diagnosis and management. Hepatogastroenterology. 2002;49:567–571. [PubMed] [Google Scholar]
- Kassir R, Debs T, Ben Amor I, Tiffet O, Blanc P, Caldwell J, et al. Management of complications following bariatric surgery: summary. Int. J. Surg. 2014;12:1462–1464. doi: 10.1016/j.ijsu.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kassir R, Tiffet O, Blanc P, Ben Amor I. Gugenheim J. Sleeve gastrectomy. A point of technique. Int. J. Surg. 2014;12:1450–1451. doi: 10.1016/j.ijsu.2014.10.021. [DOI] [PubMed] [Google Scholar]


