Figure 3.
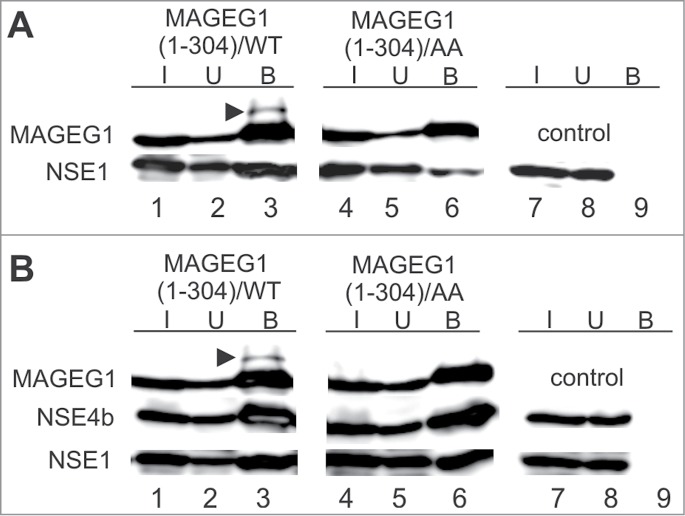
The WH/A-mediated interaction of MAGEG1 enhances the NSE1 E3-ligase activity. Either wild-type (wt, lanes 1–3) or L97A, I98A mutant (AA, lanes 4–6) of the His-S-tag-MAGEG1 construct (top panels) was co-transfected with FLAG-NSE1 alone (section A) or FLAG-NSE1 together with FLAG-NSE4b (section B) into HEK293T cells (and treated with MG132). The HEK293T cell extracts were immunoprecipitated with protein-S-beads and proteins were analyzed on western blots. In control experiments, the pTriEx4 vector (lanes 7–9) was co-transfected with NSE1 and NSE4b constructs. (A) MAGEG1 is ubiquitinated (arrowhead) in the presence of NSE1 (lane 3, top panel). The L97A, I98A (AA) mutations diminish the MAGEG1 binding to NSE1 (lane 6, bottom panel; see also Fig. S3B) and result in the disappearance of the ubi-MAGEG1 band (lane 6, top panel), suggesting the MAGEG1-NSE1 interaction-dependent ubiquitination of MAGEG1. (B) Similarly, MAGEG1 is ubiquitinated within the wt MAGEG1-NSE1-NSE4b complex (lane 3, top panel). In the presence of NSE4b, the NSE1 levels incorporated into the wild-type (lanes 1–3) and/or L97A, I98A mutant (lanes 4–6) MAGEG1-NSE1-NSE4b complexes are similar, suggesting a role of NSE4b for the stability of the complex. However, the MAGEG1/L97A, I98A protein is not ubiquitinated (lane 6, top panel) in the mutant MAGEG1-NSE1-NSE4b complex, suggesting key role of the MAGEG1 (WH/A) binding to NSE1 for its ubiquitination. Further details are in Fig. 1.
