Figure 2.
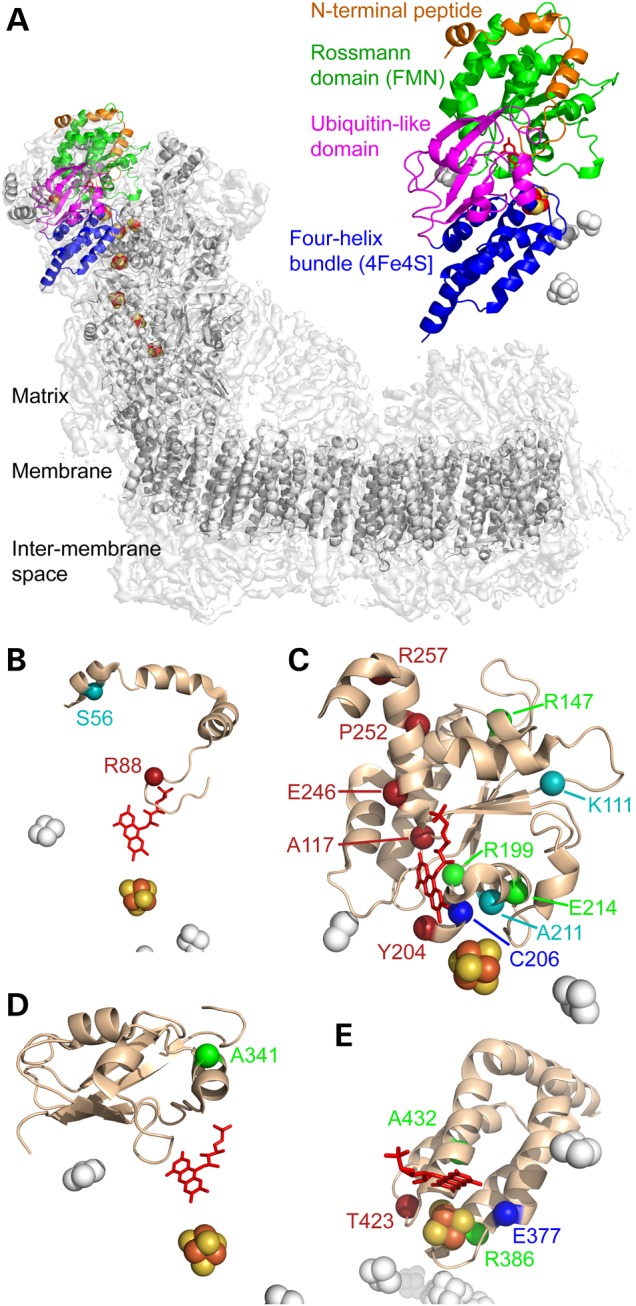
The 19 clinically identified mutations in the structure of B. taurus NDUFV1. (A) Location of NDUFV1 (the 51-kDa subunit) in the structure of complex I. The density for the whole enzyme is in light gray, with the core subunit models in dark gray and NDUFV1 in color. NDUFV1 is shown expanded on the right to illustrate its four domains. (B–E). The four domains of NDUFV1: the N-terminal peptide (B), FMN-binding Rossmann domain (C), ubiquitin-like domain (D) and FeS-binding four-helix bundle (E), with mutated residues labeled. Cyan, residues not conserved in Y. lipolytica; red, residues for which mutations produced no complex I in Y. lipolytica; blue, residues for which mutations produced complex I without any flavin in Y. lipolytica; green, residues for which mutations produced Y. lipolytica complex I with wild-type or variant properties. Figure created using 4UQ8.pdb (4).
