Figure 5.
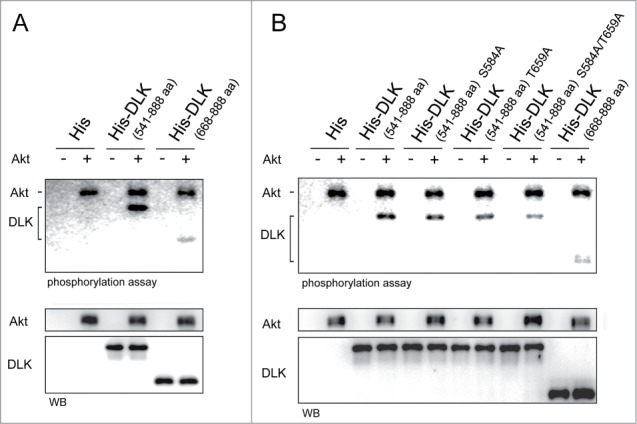
Akt phosphorylates DLK in vitro. (A) Akt phosphorylated both purified His-DLK 541-888, His-DLK 668-888 in vitro. In vitro phosphorylation assay was performed by incubation 400 ng of purified recombinant His-DLK 541-888 and His-DLK 668-888 with or without 15 ng of Akt for 15 minutes. Elutes purified from lysate of E.coli transformed with pGEX-4T vector (modified to expressed 6xHis) were used as negative control. 5 μCi of [γ-32P] ATP was added in each reaction. To quantify these proteins by Western blotting, the [γ-32P]ATP was changed to 20 μM of cold ATP for Western blotting. (B) The mutation of S584A and T659A hampered Akt phosphorylation. In in vitro phosphorylation assay, purified recombinant His-DLK 541-888, His-DLK 541-888 S584A, His-DLK 541-888 T659A, His-DLK 541-888 S584A/T659A or His-DLK 668-888 was incubated with or without of Akt. The experimental conditions were the same as in experiment (A).
