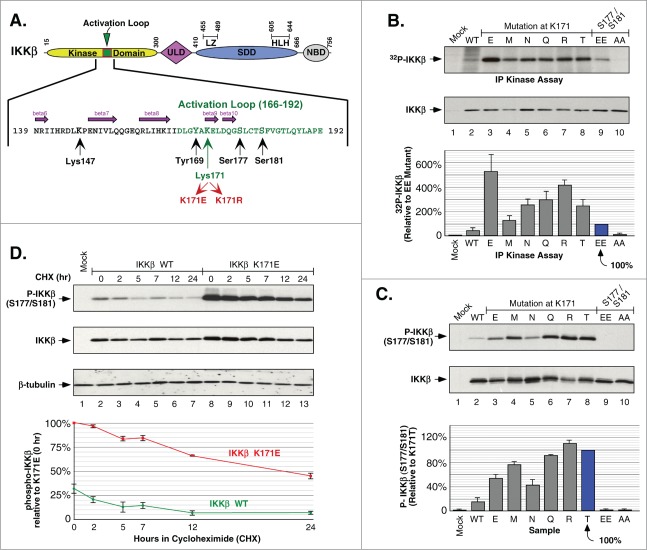
Figure 1.
Phosphorylation of IKKβ Induced by Mutations at Lys171. (A) Schematic of IKKβ with the activation loop within the N-terminal kinase domain expanded to show amino acids critical for phosphorylation and signaling. The ubiqutin-like domain (ULD), the scaffold/dimerization domain (SDD) which contains the leucine zipper (LZ) and helix-looop-helix (HLH) regions, and NEMO binding domain (NBD) are indicated. (B) IKKβ mutant proteins were expressed in HEK293 cells and the IKK complex was immunoprecipitated with IKKγ antisera and assayed for in vitro phosphorylation. Samples were separated by SDS-PAGE and detected by autoradiography. IKKβ expression is shown by immunoblotting for IKKβ (middle panel). Assays were quantitated relative to 32P incorporation of the IKKβ S177E/S181E mutant, +/− sem. (bottom) (C) HEK293T cells expressing IKKβ mutants were analyzed for activation loop serine phosphorylation using Phospho-IKKα/β antisera (top). The membrane was reprobed for IKKβ (middle panel). Serine 177/181 phosphorylation was quantitated relative to the K171T mutant, +/− sem. (bottom panel). (D) HEK293T cells expressing IKKβ WT or K171E were treated with 50 μg/ml cycloheximide (CHX) for 2, 5, 7, 12 and 24 h. Lysates were examined for IKKβ serine phosphorylation and total IKKβ as in (C) (top 2 panels). The membrane was reprobed for β-tubulin (third panel). Serine 177/181 phosphorylation was quantitated relative to K171E at time zero, +/− sem. (bottom).