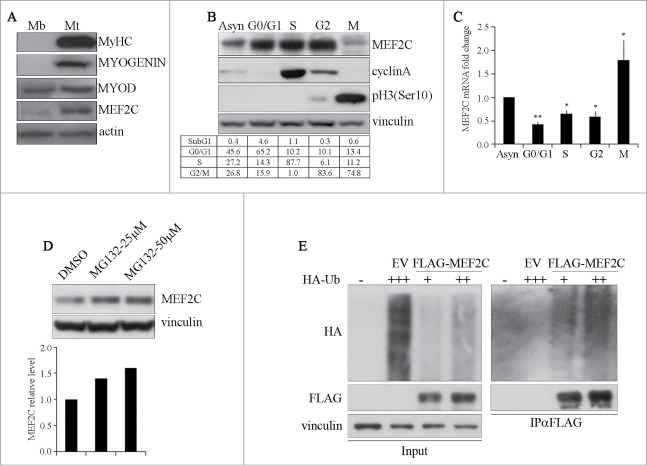
Figure 1.
MEF2C protein level is downregulated in mitosis and is targeted for degradation by the Ubiquitin-Proteasome Pathway. (A) MEF2C is expressed in proliferating myoblasts. Total protein extracts from C2 growing myoblasts (Mb) or differentiated myotubes (Mt) were analyzed by Western blot with antibodies against myosin heavy chain (MyHC), MYOGENIN, MYOD and MEF2C. Actin was used as loading control. (B) MEF2C protein level decreases in mitotic cells. C2 cells were grown asynchronously in high serum medium (Asyn), arrested in G0/G1 by incubation in methionine-depleted medium, synchronized in S by treatment with aphidicoline or incubated with Nocodazole before shake off treatment to generate a mitotic fraction (Shake off fraction; M) and a non-mitotic fraction (adherent; G2). Cell lysates obtained from synchronized cell populations were analyzed by Western blot assay with antibody against MEF2C. Antibodies specific for CYCLIN A and histone H3 phosphorylated on Ser10 (pH3(Ser10)) were used to verify the level of synchronization in S and M phases obtained with the treatments. Vinculin was used as loading control. DNA content was assessed by flow cytometry analysis, percentage of distribution in G0/G1, S and G2/M phase are reported (lower panel). (C) Analysis of Mef2C transcripts during the cell cycle. Total RNA was isolated from synchronized populations obtained as in A and Mef2c mRNA level was quantified by RT-qPCR. The ratio between Mef2c and Gapdh transcripts was calculated in each synchronized cell population. Value obtained for asynchronous C2 was arbitrarily set equal to 1. Histograms report the mean of 2 independent experiments ± SEM. * and ** represent P-values ≤ 0.05 and ≤ 0.01 respectively. (D) MEF2C is degraded by the proteasome. C2 proliferating myoblasts were incubated with increasing concentrations of the proteasome inhibitor MG132 (25 μM or 50 μM) from a stock in DMSO, the control sample received an equivalent volume of DMSO. After 4 hours of treatment cells were harvested and protein extracts were analyzed by Western blotting with anti-MEF2C specific antibody. Vinculin was used as a loading control. Lower panel shows the results of the densitometric quantification of the signals obtained with the anti-MEF2C antibody normalized for the total amount of protein. The quantity of MEF2C protein in MG132 treated cells is expressed relative to the quantity of MEF2C in the control sample (DMSO) taken as 1. (E) MEF2C is poly-ubiquitinated in proliferating myoblasts. CoIP and Western blot assays of lysates from C2 cells transiently transfected with vectors coding for FLAG-MEF2C and increasing amounts of HA-ubiquitin (HA-Ub) or Empty Vector (EV) as control. 30 hours after transfection cells were treated with MG132 and then harvested. Anti-FLAG antibody was used for immunoprecipitation (IPαFLAG), anti-FLAG and anti-HA for Western blot detection of total Input and of immunoprecipitated proteins (IPαFLAG). Western blot results shown in B, D and E are representative of 2 independent experiments showing similar profiles.