Figure 2.
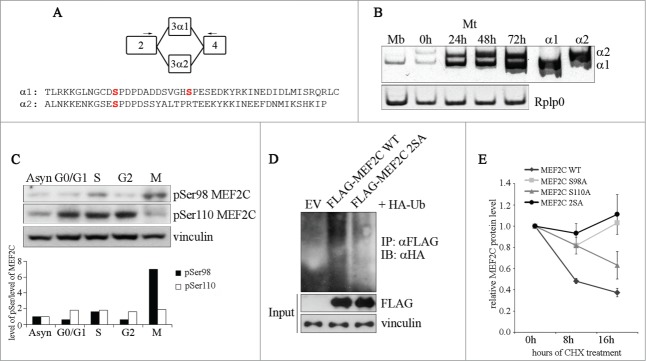
Ser98 phosphorylation of MEF2C peaks in mitosis, triggers poly-ubiquitination of the protein and reduces its stability. (A) Schematic of the MEF2C isoforms in the exon 3 region, with the location of the primers used to detect the alternate α1 and α2 exons indicated (arrows). The amminoacid sequences of the α exons of the mouse protein are indicated below. The positioning of Ser98 and Ser110 phosphoacceptor sites in α1 exon (and the residue equivalent to Ser98 in α2 exon) are highlighted in red. (B) Exclusive inclusion of the α1 exon in Mef2c transcripts in growing myoblasts. Total RNA was isolated from C2 myoblasts (Mb) or C2 cells that were differentiated for various lengths of time (0–72h). Exon expression was detected by RT-PCR using common primer (indicated in A) located on exons 2 and 4 that give rise to amplification products with different electrophoretyc mobility. Amplification of plasmid vectors containing the murine cDNAs of the MEF2C α1 and α2 splice variants were used as controls of the correct size of expected amplicons (α1 and α2 sample lanes). Rplp0 was used as endogenous control. (C). MEF2C is highly phosphorylated on Ser98 in mitotic cells. Cell lysates of asynchronous (Asyn) or synchronized cells were analyzed by Western blot with polyclonal antibodies that recognize the phosphorylated Serine 98 (pSer98) or Serine 110 (pSer110) of MEF2C. Vinculin was used as loading control. The levels of Ser98 and Ser110 phosphorylation, evaluated by densitometric scanning, were normalized to the quantity of MEF2C protein (see Fig. 1B). In the histogram below are reported the phosphorylation grades of the 2 residues in synchronized cells, expressed relative to the value obtained in asynchronous cells, taken as 1. (D). Ser98 and Ser110 phosphorylation triggers MEF2C poly-ubiquitination. C2 myoblasts were transiently transfected with expression vectors for HA-ubiquitin and FLAG-tagged wild type (WT) or not phosphorylable MEF2C 2SA mutant where Ser98 and Ser110 are substituted with alanines. Anti-FLAG antibody was used for immunoprecipitation (IPαFLAG), anti-HA for Western immunoblot detection (IB). (E) The non-phosphorylable MEF2C mutant proteins on Ser98 and Ser110 are more stable than the wild type protein. FLAG MEF2C wild type or mutated singularly on Ser98 (S98A), Ser110 (S110A) or both phosphoacceptor sites (2SA) were overexpressed in COS1 cells. After 36 h, protein synthesis was blocked with cycloheximide, the amount of FLAG MEF2C remaining at different times was checked by Western blot. Protein loading was controlled by anti- vinculin staining. The intensity of FLAG signal was quantified by densitometric analysis. The graph reports average of the intensity of MEF2C signals, relatively to the not treated sample (CHX 0h), of 2 independent experiments. Bars represent SEM. Western blot results reported in C and D are representative of 2 independent experiments that showed similar profiles.
