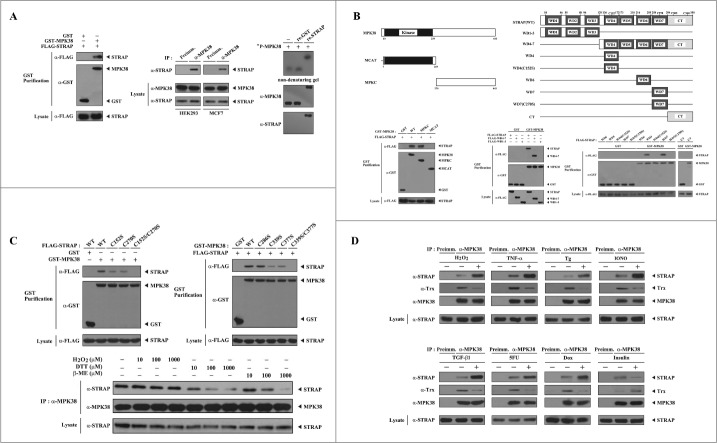
Figure 1.
Redox dependency of the interaction between MPK38 and STRAP. (A) Interaction between MPK38 and STRAP in cells. FLAG-tagged STRAP was co-transfected with glutathione S-transferase (GST)-MPK38 or vector alone (GST) into HEK293 cells. GST fusion proteins were purified on glutathione-Sepharose beads (GST Purification), and complex formation was determined by immunoblot analysis using an anti-FLAG antibody (left). To examine the endogenous interaction between MPK38 and STRAP, cell lysates from HEK293 and MCF7 cells were subjected to immunoprecipitation using either rabbit pre-immune serum (Preimm.) or anti-MPK38 antibody (α-MPK38) followed by immunoblot analysis using an anti-STRAP antibody (middle). MPK38-STRAP complex formation was also analyzed by nondenaturing PAGE (8%). Autophosphorylated recombinant MPK38 (32P-MPK38) was incubated with unlabeled recombinant GST alone or GST-STRAP, as described in “Materials and methods” (right). (B) Mapping of the binding domains involved in the MPK38-STRAP interaction. The schematic structures of wild-type (WT) and deletion constructs of MPK38 (left, upper) and STRAP (right, upper) are shown. The schematic structures of STRAP are somewhat modified compared to those reported previously.6 Numbers indicate the amino acid residues corresponding to the domain boundaries. For the mapping of MPK38 domains involved in STRAP binding, HEK293 cells were co-transfected with GST alone or GST-MPK38 constructs (WT, MCAT, and MPKC) together with FLAG-STRAP, and purified with glutathione-Sepharose beads (GST purification). The amount of STRAP proteins bound to the MPK38 constructs was determined by immunoblot analysis using an anti-FLAG antibody (lower left, top panel). The same stripped blot was re-probed with an anti-GST antibody to determine the expression of GST fusion proteins in the co-precipitates (lower left, middle panel). For the mapping of STRAP domains involved in MPK38 binding, HEK293 cells were transfected with vector alone (GST) or GST-MPK38 in combination with the indicated FLAG-STRAP deletion constructs [WD1–3, WD4–7, WD4, WD4(C152S), WD6, WD7, WD7(C270S), and CT], and cell lysates were purified using glutathione-Sepharose beads (GST purification). Complex formation was determined by immunoblot analysis using an anti-FLAG antibody (lower middle and right, top panels). (C) Involvement of cysteine residues in the MPK38-STRAP interaction. HEK293 cells transfected with the indicated expression vectors were lysed and GST precipitates were analyzed for MPK38-STRAP complex formation by immunoblot analysis using an anti-FLAG antibody (upper, top panels). To determine the redox dependency of the interaction between MPK38 and STRAP, HEK293 cell lysates were treated with the indicated concentrations of H2O2, dithiothreitol (DTT), and β-mercaptoethanol (β-ME) on ice for 0.5–1 h and then subjected to immunoprecipitation with an anti-MPK38 antibody (IP:α-MPK38). Immune complexes were analyzed for the presence of STRAP by immunoblot analysis using an anti-STRAP antibody (lower, top panel). The amount of immunoprecipitated MPK38 (lower, middle panel) and the expression levels of STRAP (lower, bottom panel) in the cell lysates were analyzed in parallel using anti-MPK38 and anti-STRAP antibodies, respectively. (D) Modulation of MPK38-STRAP complex formation by ASK1/TGF-β/p53/PDK1 signaling. HEK293 cell lysates were treated with or without the following stimuli: H2O2 (2 mM, 30 min), TNF-α (500 ng/ml, 30 min), thapsigargin (Tg: 20 μM, 30 min), ionomycin (IONO: 1 μM, 24 h), TGF-β1 (100 pM, 20 h), 5-fluorouracil (5FU: 0.38 mM, 30 h), doxorubicin (Dox: 100 ng/ml, 24 h), or insulin (100 nM, 20 min). They were then immunoprecipitated with an anti-MPK38 antibody (IP:α-MPK38), followed by immunoblotting with an anti-STRAP antibody to determine the endogenous association between MPK38 and STRAP proteins (top panels). The expression level of MPK38 in the immunoprecipitates was determined using an anti-MPK38 antibody (3rd panels). WT, wild-type; IP, immunoprecipitation; re., recombinant.