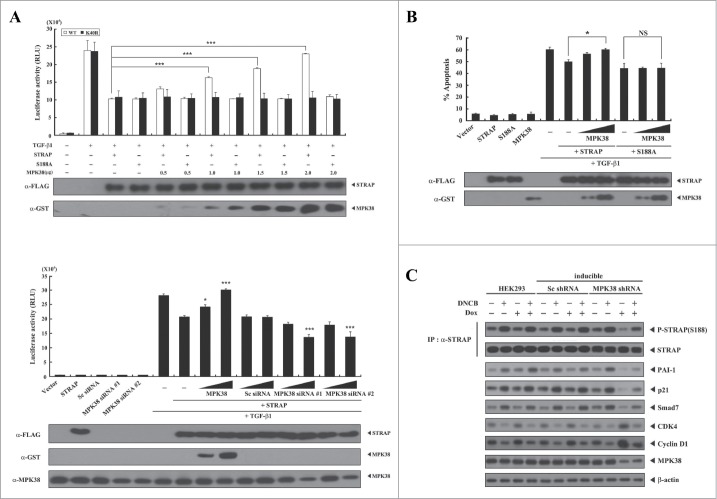
Figure 4.
Alleviation of STRAP-mediated inhibition of TGF-β signaling by MPK38-mediated STRAP Ser188 phosphorylation. (A) The effect of STRAP Ser188 phosphorylation by MPK38 on STRAP-mediated inhibition of TGF-β-induced transcription. HepG2 cells were transfected with expression vectors for wild-type (WT) and mutant (S188A) STRAP (1 μg each), 0.3 μg p3TP-Lux, increasing amounts of WT and kinase-dead (K40R) MPK38 (upper, 0.5, 1.0, 1.5, and 2.0 μg; lower, 0.6 and 1.2 μg), MPK38-specific siRNAs (100 and 200 nM), and scrambled siRNA (100 and 200 nM), as indicated, in the presence or absence of 100 pM of TGF-β1, followed by luciferase and β-galactosidase assays. Luciferase activity was measured 48 h after transfection. Results were expressed as mean SEM (upper: ***P < 0.001; lower: *P < 0.05; ***P < 0.001 versus STRAP (1 μg) in the presence of TGF-β1). (B) The effect of STRAP Ser188 phosphorylation by MPK38 on STRAP-mediated inhibition of TGF-β-induced apoptosis. HaCaT cells were transfected with WT and mutant (S188A) STRAP (1 μg each), green fluorescent protein (2 μg), and increasing amounts of expression vectors for WT MPK38 (1 and 2 μg) in the presence or absence of TGF-β1 (100 pM). Apoptotic cell death was determined as described in Fig. 3B. Results were expressed as mean SEM (*P < 0.05; NS, not significant). (C) The effect of STRAP Ser188 phosphorylation by MPK38 on the expression of TGF-β target genes. HEK293 cells expressing inducible MPK38 or scrambled shRNA treated with or without 1-chloro-2,4-dinitrobenzene (DNCB) and doxycycline (Dox), as described in Fig. 3C, were lysed and subjected to immunoblot analysis using anti-PAI-1, anti-p21, anti-Smad7, anti-CDK4, anti-Cyclin D1, anti-MPK38, and anti-β-actin antibodies. Sc, scrambled.