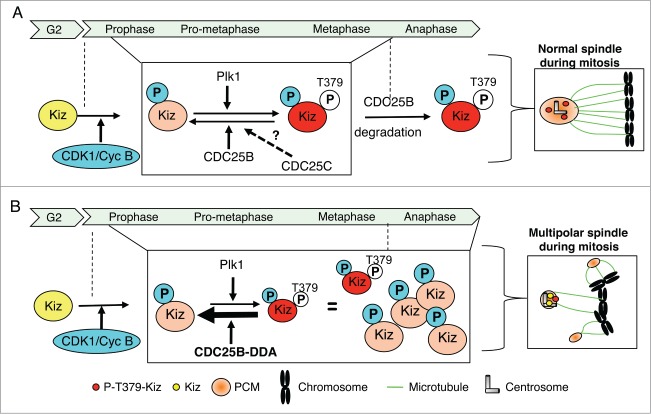
Figure 9.
Proposed model for the regulation of Kiz phosphorylation during mitosis. (A). During mitosis, phosphorylation level of Kiz results from CDK1/Cyc B kinase activity and a balance between Plk1 kinase and CDC25B phosphatase activities. At the entrance into mitosis Kiz is phosphorylated by CDK1/Cyc B, a priming phosphorylation that facilitates its phosphorylation on Thr379 by Plk1. Until metaphase-anaphase transition, phosphorylation state of Thr379 results from a balance between activities of Plk1 and CDC25B (and maybe CDC25C) to finely control the equilibrium between expansion and stabilization of the PCM around the centrosomes. At the metaphase-anaphase transition, when CDC25B is degraded by the proteasome, Kiz is fully phosphorylated on Thr379 by Plk1. (B). During mitosis, if a defect in proteasome-dependent degradation of CDC25B occurs, such as when the CDC25B-DDA mutant is expressed, the high level of CDC25B maintains Kiz in an hypo-phosphorylated form that hinders its ability to stabilize PCM around the centrosomes during all phases of mitosis, resulting into the formation of multipolar spindles.