Figure 1.
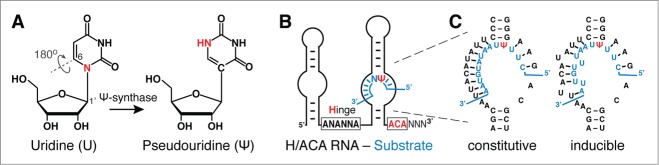
Pseudouridylation, guide RNA, and guide mechanism. (A) Chemical structure of uridine (U) and pseudouridine (Ψ). The 180° rotation of the base frees up the N-glycosidic nitrogen in U as an additional hydrogen donor in Ψ (red). The numbering of base and ribose positions for potential nucleophilic attacks by a conserved aspartate of the Ψ-synthase is indicated. Note the aspartate is highlighted in yellow in the dark green catalytic domain of the Ψ-synthase (Fig. 2, inset). (B) Schematic of an H/ACA guide RNA hybridized to a substrate RNA (blue) in its 3′ pseudouridylation pocket. The hinge region and ACA conserved sequence elements responsible for the H/ACA name are highlighted (red; N = any nucleotide). (C) Example of the pseudouridylation pocket of the yeast H/ACA RNA snR81 hybridized to its substrate RNA (blue). Perfect Watson-Crick base-pairs between the guide sequence and the substrate RNA result in constitutive pseudouridylation at the target site (left panel, constitutive pseudouridylation). In contrast, if the guide sequence forms imperfect base-pairs (there are 2 mismatches) with the substrate RNA, pseudouridylation occurs only under stress (nutrient-deprivation) conditions (right panel, inducible pseudouridylation).
