Figure 7.
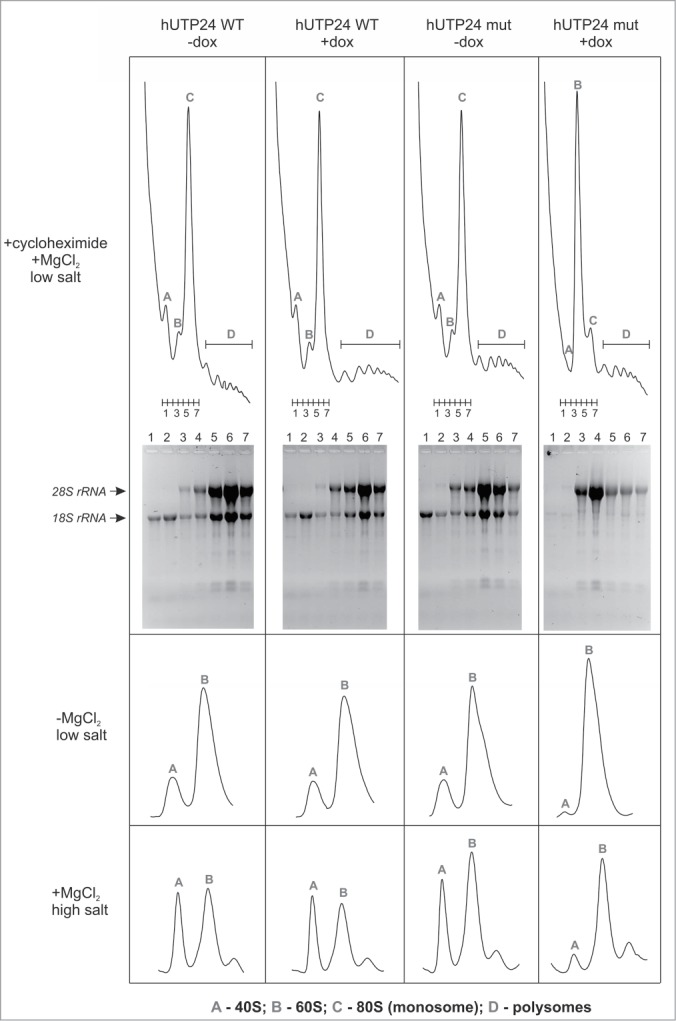
Mutation in the hUTP24 putative catalytic site leads to a ribosome biogenesis defect. Analysis of polysome and ribosome subunits profiles was conducted by preparing native cytoplasmic extracts from model cell lines, grown in the absence (“−dox”) or presence (“+dox”) of doxycycline, using buffers with cycloheximide (top panel), lacking magnesium (middle panel), or containing salt at high concentration (bottom panel), and separated by centrifugation in linear sucrose gradients. Graphs show distribution of absorbance at 254 nm from the top (left) to the bottom (right). Peaks corresponding to individual subunits (40S and 60S), monosomes (80S) and polysomes are indicated. In the experiment performed using cycloheximide, 7 fractions were collected from each gradient (numbered 1–7), as indicated below the graphs. RNA was then isolated from these fractions and separated in denaturing agarose-formaldehyde gels. The bottom part of the panel demonstrates results of the electrophoretic analysis. Expression of hUTP24 mut leads to diminished levels of the 40S subunit and monosomes, which contain reduced amounts of both 18S and 28S rRNA.
