Fig. 3.
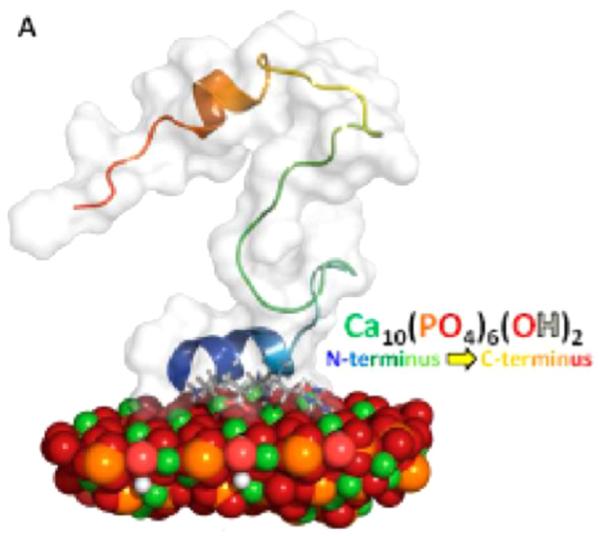
A representative lowest energy structure of statherin bound to the 100 face of HAP, obtained with RosettaSurface and consistent with SSNMR constraints. The N-terminus is helical and associated with the surface, while the C-terminus folds back on itself and is thought to serve a role in lubrication of bacterial binding. Reproduced with permission from Ref. [44]. Copyright 2014 American Chemical Society.
