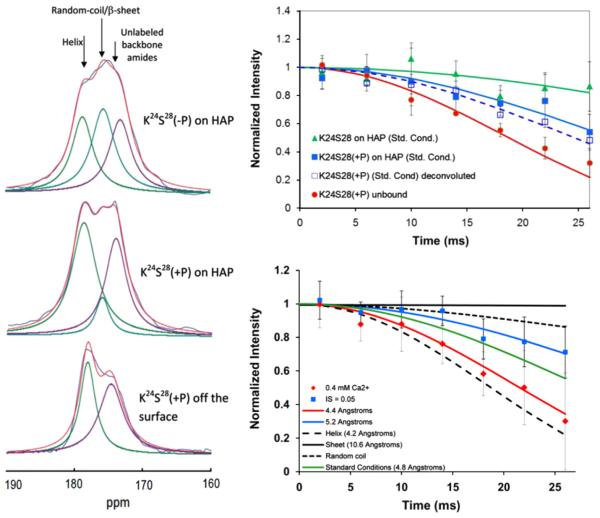
Fig. 8.
Left: The K24S28 region of LRAP is a highly sensitive structural region. This sensitivity can be observed in the 1D spectra which show multiple resonances for the 13C′ of K24 whose relative intensities change as a function of the conditions. Multiple resonances are only observed for this residue in the protein, further evidence of the structural uniqueness of this region. Right, top: The structural sensitivity is quantitatively shown with REDOR studies, with distances ranging from 4.2 Å for the unbound, lypohilized protein (the distance expected for an ideal helix), to 5.7 Å for the bound, unphosphorylated protein, a very extended or random coil structure. Bottom, right: Other conditions such as the Ca2+ concentration when binding, or binding to carbonated HAP instead of HAP, also influence the structure. Reproduced with permission from Ref. [55] and Ref. [56], Copyright 2013 American Chemical Society.