Abstract
RNA-helicases are proteins required for the unwinding of occluding secondary RNA structures, especially at low temperatures. In this work, we have deleted all 4 DExD-box RNA helicases in various combinations in the Gram-positive pathogen Listeria monocytogenes. Our results show that 3 out of 4 RNA-helicases were important for growth at low temperatures, whereas the effect was less prominent at 37°C. Over-expression of one RNA-helicase, Lmo1450, was able to overcome the reduced growth of the quadruple mutant strain at temperatures above 26°C, but not at lower temperatures. The maturation of ribosomes was affected in different degrees in the various strains at 20°C, whereas the effect was marginal at 37°C. This was accompanied by an increased level of immature 23S rRNA precursors in some of the RNA-helicase mutants at low temperatures. Although the expression of the PrfA regulated virulence factors ActA and LLO decreased in the quadruple mutant strain, this strain showed a slightly increased infection ability. Interestingly, even though the level of the virulence factor LLO was decreased in the quadruple mutant strain as compared with the wild-type strain, the hly-transcript (encoding LLO) was increased. Hence, our results could suggest a role for the RNA-helicases during translation. In this work, we show that DExD-box RNA-helicases are involved in bacterial virulence gene-expression and infection of eukaryotic cells.
Keywords: bacterial infection, DExD-box RNA-helicases, hly, Listeria monocytogenes, LLO, translation
Introduction
RNA-helicases are proteins involved in several steps of the maturation of RNA-molecules. Most RNA-helicases are either part of the DExH-family of processive RNA-helicases or the DExD-family of non-processive RNA-helicases where DExH or DExD refer to the consensus amino-acid sequence of the ATP-binding catalytic center. In eukaryotes, RNA-helicases have been assigned roles in RNA-splicing and editing, translation and mRNA degradation.1 Also, RNA-helicases are important for appropriate ribosomal maturation and spliceosomal formation.1 In bacteria, most attention has been drawn to the DExD-family of RNA-helicases. Generally, this family of RNA-helicases are involved in ribosomal maturation and mRNA decay.2-4 The function of RNA-helicases of the DExH-family is less clear. In Escherichia coli, DExD-box RNA-helicases have been shown to be an important component of RNA degradosomes, protein complexes essential for RNA degradation and maturation.5,6 RNA-helicases in Gram-positive bacteria are often associated with RNA degradosomes.3,7,8 Interestingly, Bacillus subtilis and Listeria monocytogenes both harbor 4 DExD-box RNA-helicase genes whereas Staphylococcus aureus only has one (Fig. 1). The enzymatic core region of each DExD-box RNA-helicase is relatively conserved, resembling RecA, whereas the C-terminal part varies extensively.9
Figure 1.

Schematic representation of DExD-box RNA-helicases in some Gram-positive species. The length of L. monocytogenes DExD-box RNA-helicases are shown together with the schematix drawing of the core-region (box) and the C-terminal part. B. subtilis, B. cereus and S. aureus RNA-helicase homologs are shown to the right. *CshE of B. cereus is most similar to Lmo0866 (e−128, BLAST homology score).
So far, only a few bacterial RNA-helicases have been implicated in virulence. HrpA, a DEAH-box RNA-helicase of Escherichia coli has been shown to be required for proper fimbrial processing.10 More recently, the DEAH-box RNA-helicase HrpA of Borrelia burgdorferi was shown to be essential for mouse infectivity and tick transmission.11,12 To our knowledge, only one DExD-box RNA-helicase, CshA of Staphylococcus aureus, has been shown to be involved in virulence factor expression.13,14 It was shown that absence of CshA increased the stability of agr mRNA and hence hemolysis, whereas S. aureus biofilm formation was inhibited.
Listeria monocytogenes is a Gram-positive, intracellular food-borne pathogen, able to cause severe infections in immunocompromised individuals.15,16 Infection of human cells follow a strict temporal and spatial pattern, requiring the concerted action of several virulence factors.16 Among these are different adhesins (internalins) required for bacterial attachment to eukaryotic cells; a haemolysin (Listeriolysin O) essential for escape from the phagosome and an actin-polymerization factor (ActA) needed for cell to cell spread.16 Expression of these virulence factors are controlled by the transcriptional regulator PrfA.17-19 We have previously shown that one DExD-box RNA-helicase, Lmo1722, is important for ribosome maturation and binds the 50S ribosomal subunit through its C-terminal domain.20 Also, the Listeria RNA-helicases appear to be most important if the bacterium faces different stress-conditions, such as low temperature.21,22
In this work, we have characterized the function of all DExD-box RNA-helicases in the Gram-positive pathogen Listeria monocytogenes by combining different knock-out mutants. Our results indicate a hierarchy among the RNA-helicases, where Lmo1450 appears most important considering growth, motility and ribosome maturation at low temperatures (from 16 to 26°C). At permissive temperature (37°C), the RNA-helicases are less important for growth, 23S rRNA precursor processing, ribosomal maturation, but are still required for appropriate expression of virulence factors and infectivity. We also observe a putative function of RNA-helicases during translation.
Results
A L. monocytogenes strain lacking all DExD-box RNA helicases is viable
During the course of this work, it was shown that 3 out of 4 RNA-helicases in Listeria monocytogenes were important for maximal growth and motility.23 We observed similar phenotypes in our single knockout mutants, with the largest effect observed at low temperatures (data not shown, Table 1, Figs. S1–S3). The individual knock-out mutants could be complemented by expression in trans, eliminating the possibility of polar effects in accordance with previous work (23 and data not shown). In order to determine the overall function of RNA-helicases, strains lacking 2, 3 or all 4 RNA-helicases were constructed. These strains were examined for their growth abilities at different temperatures in liquid media (Table 1, Figs. S1–S3). From the results, it was clear that the RNA-helicases Lmo1722 and Lmo1246 play a minute, if any, role during growth at 37°C, since the growth-rate of the Δlmo1246, Δlmo1722 double mutant strain was similar to the wild-type, whereas a strain lacking only Lmo1722 showed a reduced growth rate at low temperatures as shown previously (Table 1, Figs. S1–S3 20). Absence of either Lmo0866 or Lmo1450 prolonged the generation time at all temperatures, although the reduced growth rates of the various RNA-helicase strains were not followed by an increased bacterial death (Table 1, Figs. S1–S3). Interestingly, absence of Lmo1450 in any combination (single, double, triple or quadruple mutant) lead to an almost identical growth rate of the bacterial strains at 37°C, indicating that the reduced growth-rate at that temperature was to a large degree due to the lack of Lmo1450 (Table 1, Fig. S3). When over-expressing the different RNA-helicases in the quadruple mutant at different temperatures, we observed a gradual improvement of growth in the following order of importance: Lmo1450 > Lmo0866 > Lmo1722 > Lmo1246 (Fig. 2). Although not reflecting a physiological condition, the results indicate that high levels of Lmo1450 can overtake the role of the other RNA-helicases and re-establish growth of the quadruple mutant at temperatures higher than 26°C. At 16°C, overexpression of Lmo1450 was only partially able to overcome the growth impairment of the quadruple mutant strain, indicating that the other RNA-helicases have specific functions at lower temperatures that excess Lmo1450 is unable to suppress.
Table 1.
Generation times (g ± SD) and viability (dilution test on agar plates) of various L. monocytogenes RNA helicase mutants at 16°C, 25°C and 37°C respectively
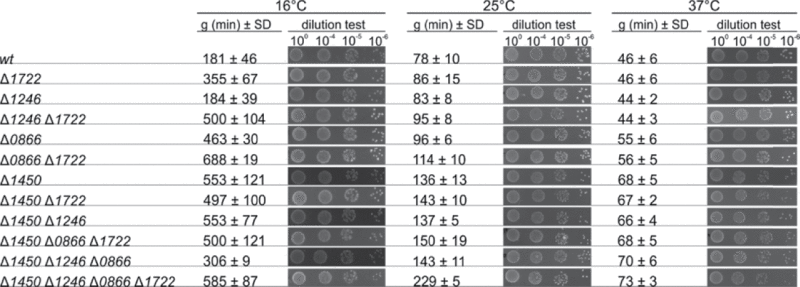 |
Figure 2.
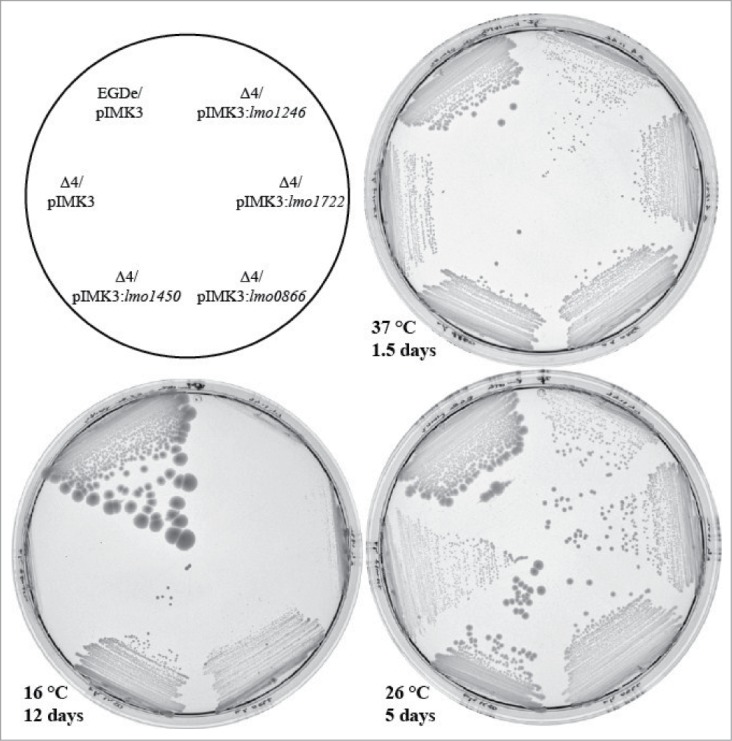
Growth of the quadruple RNA helicases mutant strain complemented with each helicase gene. Wild-type (EGDe) carrying the vector control pIMK3, or quadruple mutant (Δ4) strains carrying the RNA helicase genes of lmo1246, lmo1722, lmo0866, lmo1450, or pIMK3, respectively, were grown on BHI agar-plates at indicated temperatures and for designated times in presence of 1 mM IPTG.
Absence of RNA-helicases affect ribosomal maturation
In many cases, absence of RNA-helicases affects ribosomal maturation, especially at low temperatures.24,25 We have previously observed that a L. monocytogenes strain lacking the RNA-helicases Lmo1722 had a reduced fraction of 50S ribosomal subunits.20 This was in line with a C-terminal dependent association of Lmo1722 with the 50S subunit.20 Here, we were interested to examine ribosomal maturation in strains lacking different combinations of RNA-helicases. Absence of Lmo1246 did not affect the overall ribosomal maturation at 20°C, whereas absence of Lmo1722, Lmo0866 or Lmo1450 showed a clear effect (Fig. 3A). The lack of Lmo1722 or Lmo0866 decreased the amount of 50S subunits and 70S ribosomes. Absence of Lmo1450 on the other hand lead to an increased level of 50S subunits and decreased 70S levels. The quadruple mutant strain essentially followed the ribosomal maturation pattern of the Δlmo1450 strain, furthermore emphasizing the important physiological role of Lmo1450. We were unable to detect any differences in the appearance of intermediate ribosomal subunits in the different strains (Fig. 3A). This was in contrast to E. coli strains lacking the RNA-helicases SrmB and CsdA which displayed an increased level of 40S subunits.26,27 The absence of RNA-helicases gave a much less pronounced effect in ribosomal maturation at 37°C, although a similar trend compared with 20°C was observed (Fig. 3B). Importantly, the amount of mature 70S subunits was similar in all strains at 37°C, suggesting that initial loading of 70S ribosomes on mRNA was not affected. It is therefore of interest to note that the amount of polysomes (transcript harboring more than one translating ribosome) was almost absent in the Δlmo0866 strain, the Δlmo1450 strain and the quadruple mutant strain as compared with the wild-type strain at 37°C (Fig. 3B).
Figure 3.
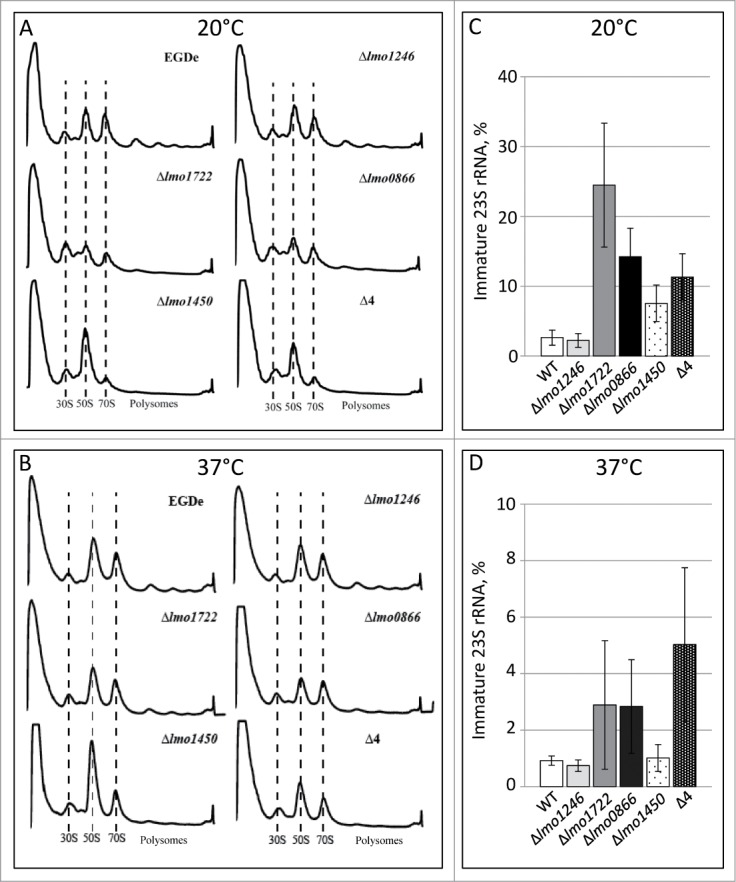
Ribosomal maturation and 23S rRNA processing at different temperatures. Ribosomal profiles of indicated strains were generated by the measurement of light absorbance at A254 of lysates fractionated in sucrose gradients at 20°C (A) or at 37°C (B). 30S, 50S, 70S and polysome profiles are shown below. Indicated strains were grown at 20°C (C) or 37°C (D) before RNA was isolated and the amount of non-processed compared with processed 23S rRNA precursor transcript (%) was determined.
The DExD-box RNA-helicases are important for 23S rRNA precursor processing
In L. monocytogenes, the immature 23S rRNA harbor a 160 nucleotide long 5´precursor. Previously, it was shown that 23S rRNA processing required Lmo1722.20 Surprisingly, direct contact between the 50S subunit and Lmo1722 through its C-terminal part was redundant for 23S rRNA processing activity. We were interested to examine if impaired ribosomal maturation observed in the different RNA-helicase mutants was accompanied by a deficiency of 23S rRNA precursor processing. Through a primer extension analysis, it was observed that the strains displaying a reduced ribosomal maturation also showed an increased level of immature 23S rRNA precursor. At 20°C, the largest effect was observed in the Δlmo1722 mutant strain (∼25% immature 23S rRNA), although all other mutants, except the Δlmo1246 strain, also showed a reduced ability to process the 23S rRNA precursor (Fig. 3C). At 37°C, a similar pattern to 20°C was observed, while at this temperature, the quadruple mutant strain exhibited the most prominent increase of immature 23S rRNA precursors (Fig. 3D).
RNA-helicases affect virulence gene expression
Recently, it was shown that the DExD-box RNA helicase CshA was involved in S. aureus hemolysis and biofilm formation through modulation of the agr-mRNA stability.13,14 We were therefore interested to examine the putative role of the Listerial DExD/box RNA-helicases in virulence and chose to investigate the levels of 2 major virulence factors, LLO and ActA. Expression of the actin-polymerization responsible factor ActA was decreased in all mutant strains, except the Δlmo1246 strain, compared with the wild-type strain (Fig. 4A). Also, the level of the phagosome lysing haemolysin LLO was measured. Absence of Lmo1722 and Lmo0866 decreased LLO expression by approximately 50% when relating to wild-type levels (Fig. 4A). Essentially no difference in LLO expression could be observed in the Δlmo1450 strain compared with the wild-type. This suggests that the reduced LLO-levels in the other RNA-helicase mutants were not caused by a decreased growth rate since the Δlmo1450 strain displayed the largest growth impairment at 37°C (Table 1, Figs. S1-S3). The LLO level was slightly reduced in the quadruple mutant strain compared with the wild-type strain. Surprisingly, the hly mRNA levels (encoding LLO) were increased in the quadruple mutant strain (Fig. 4B). Thus, the increased hly transcript levels and decreased LLO-levels in the quadruple mutant strain could indicate a requirement for RNA-helicases during LLO translation. Since CshA in S. aureus was shown to destabilize the agr-transcript,13,14 we were interested to examine if the hly transcript stability was affected in the quadruple mutant strain. No detectable differences in hly-transcript stability between the wild-type and the quadruple mutant strain could however be observed, suggesting that the increased level of hly-transcript observed in a quadruple mutant strain was due to elevated transcription and not higher stability (Fig. 5). Almost all virulence factors in L. monocytogenes are controlled by one single regulator, PrfA, a transcriptional activator of the Crp/Fnr family.17,18,28 Since the levels of the virulence factors LLO and ActA were reduced in the quadruple mutant strain, it could be hypothesized that the PrfA-levels were affected. However, the prfA transcript amount and stability did not differ between the wild-type and the quadruple mutant strain nor did the PrfA protein levels (Figs. 4A and 5).
Figure 4.
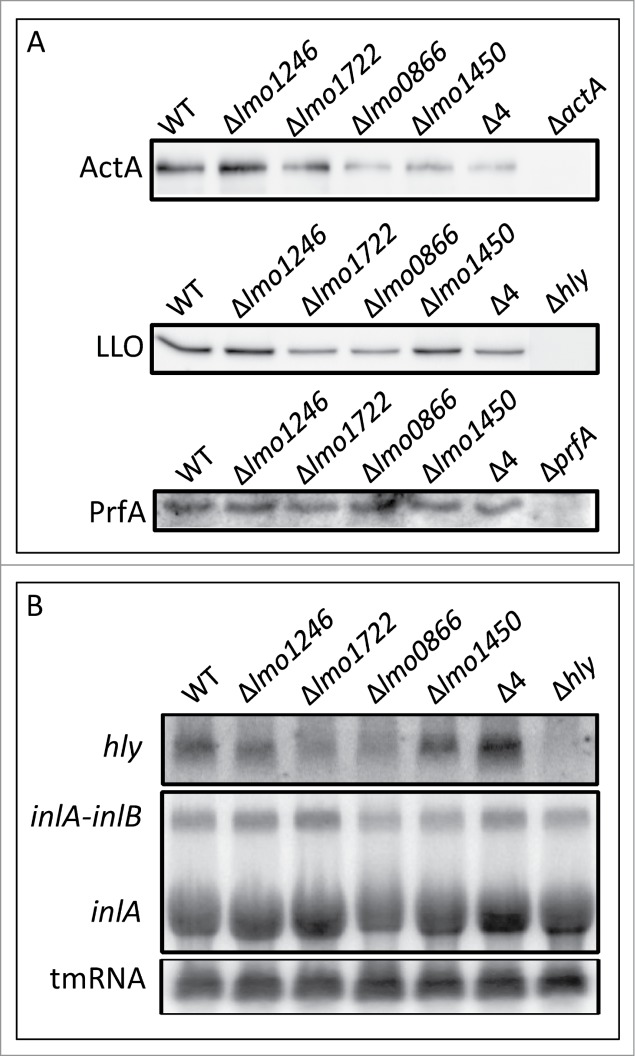
Expression levels of virulence-related genes in RNA helicase mutant strains. (A) Indicated strains were grown and levels of specified proteins were analyzed using Western blotting: ActA was extracted by boiling bacterial cells in 1×Laemmli sample loading buffer, LLO was analyzed in culture supernatants after trichloracetic acid precipitation, whereas PrfA was detected in whole bacterial lysates. (B) Indicated strains were grown and total RNA was isolated before separation on an agarose gel and RNA gel blot. Radioactively labeled DNA-probes specifically recognizing hly, inlAB and tmRNA transcripts were used for detection.
Figure 5.
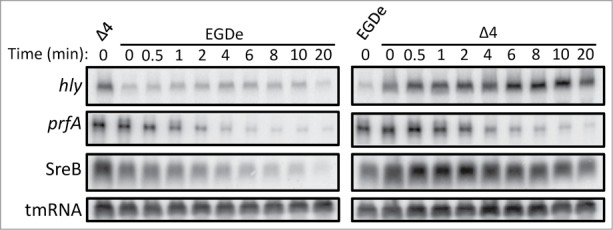
Transcript stability of selected genes in the WT and quadruple RNA helicase mutant L. monocytogenes strains. The stability of hly, prfA, SreB and tmRNA transcripts, respectively, was analyzed by Northern blotting of RNA isolated from wild-type (EGDe) or quadruple mutant (Δ4) strains harvested at indicated time points (in minutes) after rifampicin addition. Radioactively labeled DNA-probes specifically recognizing hly, prfA, SreB and tmRNA transcripts were used for detection.
RNA-helicases are involved in bacterial uptake into eukaryotic cells
Do the altered levels of LLO and ActA observed in the different RNA-helicase mutant strains affect the infection capability? To address this, a cell-infection experiment was performed where the different strains were allowed to infect cultured Caco2-cells. To avoid indirect intracellular growth-related effects between the different RNA-helicase mutants, we only examined the uptake of the bacteria (early time-point – 1 hour post-infection) and not intracellular replication. Surprisingly, the Δlmo1450 and the quadruple mutant strains showed a significantly increased uptake compared with the wild-type strain, whereas absence of Lmo0866 and Lmo1722 did not show any differences (Fig. S4). Unexpectedly, absence of Lmo1246 displayed a significantly reduced uptake capacity as compared with the wild-type (Fig. S4). It could be hypothesized that the increased uptake of the Δlmo1450 strain and the quadruple mutant strain could be due to an increased activity of the stress-sigma factor σB which in turn activates expression of the L. monocytogenes cell-infection dependent adhesins InlA and InlB.29 However, no difference in inlAB transcript levels could be observed between wild-type and the RNA-helicase mutant strains (Fig. 4B).
Discussion
RNA-helicases are proteins essential for the function of various RNA-species. In this work, we highlight roles for L. monocytogenes DExD-box RNA-helicases during growth, ribosomal maturation and virulence. Previous work has shown individual roles of L. monocytogenes RNA-helicases during growth and at different stress-responses.20,21,23 Here, we show that a L. monocytogenes strain lacking all RNA-helicases is still viable at all temperatures tested and only show approximately a 50% longer generation time compared with the wild-type at 37°C (Table 1, Figs. S1-S3). Over-expression of Lmo1450, but none of the other RNA-helicases, was shown to be sufficient for restored growth of the quadruple mutant strain at temperatures above 26°C (Fig. 2). However, higher amounts of Lmo1450 could not re-establish growth of the quadruple mutant strain at 16°C, indicating specific roles of the other RNA-helicases at low temperatures. RNA-helicases in other Gram-positive bacteria have also been shown to be important for growth at and/or adaptation to low temperatures 13,30-34 but also at other stress-conditions.31 As in Listeria, absence of all RNA-helicases in B. subtilis (Δ4 quadruple mutant strain), drastically prolonged the generation time at low (16°C) temperatures.33 Another Gram-positive psychrotolerant foodborne pathogen, closely related to L. monocytogenes is Bacillus cereus. B. cereus harbors 5 DExD-box RNA-helicases of which 3 (CshA, CshB and CshC, corresponding to Lmo0866, Lmo1450 and Lmo1722, respectively) have been shown to be important for stress-adaptation and growth at low temperatures (Fig. 1, 30-32 ). CshD and CshE of B. cereus (corresponding to Lmo1246 and the core region of Lmo0866, respectively), appear to be redundant at these stress-conditions.30,31 Whether or not the RNA-helicases of B. cereus contribute to virulence gene expression and pathogenesis remain to be analyzed.
The functional differences of the RNA-helicases were reflected by their involvement in the maturation of ribosomal subunits, especially evident at low temperatures (Fig. 3). This is a common trait in bacterial strains lacking RNA-helicases.25 As also observed in B. subtilis RNA-helicase mutants, we were unable to detect any increase in intermediate subunits (between 30S and 50S) when analyzing the ribosomal maturation of the quadruple mutant.33 Such intermediary ribosomal subunits (40S) have been identified in E. coli RNA-helicase mutants.2 This could suggest differences in the assembly of ribosomes in Gram-positive and Gram-negative bacteria as has been proposed earlier.33 A ribosomal maturation difference was also observed at 37°C, where absence of Lmo0866 or Lmo1450 had opposite effects on 50S subunit levels (Fig. 3). We observe a decreased processing of the 23S rRNA precursor, particularly in a strain lacking Lmo1722, in agreement with previous findings.20 In E. coli, absence of SrmB and CsdA (DeaD) has been shown to affect processing of the 23S rRNA precursor.26,27 However, the mechanism by which the RNA-helicases function in 23S rRNA processing might be different since in E. coli the 23S rRNA precursor is only 15 nucleotides longer than the mature form, compared with 160 nucleotides longer precursor in Listeria.
The Δlmo0866, the Δlmo1450 and the quadruple mutant strains all showed reduced levels of polysomes in their ribosomal fractions at both 20 and 37°C (Fig. 3). This has previously been shown in a strain lacking Lmo1722 at 16°C.20 Absence of the E. coli RNA-helicases SrmB and CsdA (DeaD) also decrease the number of polysomes, but only at low temperatures (30 and 20°C, respectively).26,27 The reduced level of polysomes in Listeria could possibly explain to the reduced translation of hly mRNA that we observed at 37°C (i.e. the level of hly mRNA was higher, but the amount of LLO protein was reduced in the quadruple mutant as compared with the wild-type - Fig. 4). Whether this reflects a decreased translation capacity, less functional 70S ribosomes or increased mRNA turnover in strains lacking DExD-box RNA-helicases remains to be investigated. Talking against the latter 2 alternatives were the following findings: 1. The level of 70S monosomes (single ribosomes loaded on the transcript) was almost identical in the wild-type and the quadruple mutant strain (Fig. 3B). 2. The high mRNA/low protein expression pattern observed for hly/LLO in the quadruple mutant was not general for all gene-products (as it should be if the 70S ribosomes in this strain would be less functional), since the ratio of PrfA protein: prfA transcript was similar between the wild-type and the quadruple mutant strain (Figs. 4 and 5). 3. The hly mRNA stability was similar in the wild-type and quadruple mutant strains (Fig. 5). Recently, a role for the E. coli RNA-helicase CsdA (DeaD) was observed during translation.35 In that study, the author could show that the RNA-helicase stimulated translation of UvrY, most likely by making the uvrY-transcript accessible for ribosomes.35 It remains to be investigated whether the listerial RNA-helicases also make target mRNAs more available for ribosomes or if they act by other mechanism(s). It has been suggested that CsdA (DeaD) stimulate rpoS translation at low temperatures in a mechanism involving the RNA-chaperone Hfq and the small RNA DsrA.36 In this paper, we also observe a connection between RNA-helicases and a small regulatory RNA: the trans-acting riboswitch SreB, which inhibits prfA-translation by binding to its 5′-UTR, shows a higher level in the quadruple mutant strain, most probably by an increased stability in that as compared with the wild-type strain (Fig. 5.37)
Recently, CshA, a DExD-box family RNA-helicase was shown to negatively regulate hemolytic activity and positively control biofilm formation in Staphylococcus aureus.13,14 To our knowledge, no bacterial DExD-box RNA-helicase has previously been shown to be important during bacterial infection of eukaryotic cells. In this work, we show that several Listerial RNA-helicases are required for maximal expression of the virulence factors LLO and ActA, whereas expression of the virulence regulator PrfA was essentially unaffected (Fig. 4). This would suggest that the RNA-helicases act at the level of PrfA post-translational activation, possibly by affecting the nature of the yet unknown co-factor that is supposed to increase PrfA activity.18,19 Surprisingly, the reduction in virulence factor expression in these strains was not accompanied by decreased uptake into eukaryotic cells (Fig. S4). Instead, the uptake of these mutant strains into cultured cells became higher as compared with the wild-type strain. The reason for this apparent contradiction is unknown, but it was not due to an increased level of the mRNA encoding the InlA and InlB adhesins (Fig. 4B).
Materials and Methods
Strains and plasmid construction
E. coli and L. monocytogenes strains are listed in Table 2. E. coli were grown in LB and L. monocytogenes in BHI at indicated temperatures, unless otherwise noted. Where needed, antibiotics were included in the growth media at these final concentrations: carbenicillin 100 μg/ml; kanamycin 50 μg/ml; nalidixic acid 50 μg/ml; colistin sulfate 10 μg/ml. Cloning was performed using standard techniques.38
Table 2.
Bacterial strains used in this study.
| Strain | Relevant genotype/phenotype | Reference |
|---|---|---|
| Escherichia coli DH5α | Cloning host | 51 |
| Escherichia coli S17-1 | E. coli strain used for conjugative plasmid transfer to L. monocytogenes | 52 |
| Listeria monocytogenes EGDe | Wild-type Listeria monocytogenes | 53 |
| Δhly | EGDe deleted of hly | 32 |
| ΔactA | EGDe deleted of actA | 32 |
| ΔprfA | EGDe deleted of prfA | 22 |
| Δlmo1722 | EGDe deleted of lmo1722 | 20 |
| Δlmo1246 | EGDe deleted of lmo1246 | This study |
| Δlmo0866 | EGDe deleted of lmo0866 | This study |
| Δlmo1450 | EGDe deleted of lmo1450 | This study |
| Δlmo1246 Δlmo1722 | EGDe deleted of lmo1246 and lmo1722 | This study |
| Δlmo0866 Δlmo1722 | EGDe deleted of lmo0866 and lmo1722 | This study |
| Δlmo1450 Δlmo1722 | EGDe deleted of lmo1450 and lmo1722 | This study |
| Δlmo1450 Δlmo1246 | EGDe deleted of lmo1450 and lmo1246 | This study |
| Δlmo1450 Δlmo0866 Δlmo1722 | EGDe deleted of lmo1450, lmo0866 and lmo1722 | This study |
| Δlmo1450 Δlmo1246 Δlmo0866 | EGDe deleted of lmo1450, lmo1246 and lmo0866 | This study |
| Δ4, quadruple mutant | EGDe deleted of lmo1722, lmo1246, lmo0866 and lmo1450 | This study |
| Δ4/pIMK3:lmo0866 | Quadruple mutant strain overexpressing lmo0866 | This study |
| Δ4/pIMK3:lmo1246 | Quadruple mutant strain overexpressing lmo1246 | This study |
| Δ4/pIMK3:lmo1450 | Quadruple mutant strain overexpressing lmo1450 | This study |
| Δ4/pIMK3:lmo1722 | Quadruple mutant strain overexpressing lmo1722 | This study |
| Δ4/pIMK3 | Quadruple mutant strain carrying pIMK3 vector control | This study |
| EGDe/pIMK3 | Wild-type strain carrying pIMK3 vector control | This study |
Flanking regions of lmo1246, lmo0866 and lmo1450 were amplified with primers listed in Supplementary Table S1. The PCR products were cloned in tandem into the pMAD vector, creating plasmids pMAD1246, pMAD0866 and pMAD1450 respectively (Supplementary Table S2). The constructs were sequenced to ensure wild-type sequences of clones. Gene deletions were then performed as described previously.39 Multiple deletion mutants were constructed in a sequential manner. Complementation of helicases deletions was done by cloning of the helicase genes (using lmo0866 F and R; lmo1246 F and R; lmo1450 F and R oligonucleotides, respectively) into an IPTG-inducible pIMK3 vector,40 creating pIMK3:lmo0866; pIMK3:lmo1246 and pIMK3:lmo1450, respectively. The pIMK3:lmo1722 was created previously.20 The resulting constructs were transferred to L. monocytogenes by conjugation with E. coli S17-1 strain carrying these plasmids.41 Transconjugants were selected by plating on BHI plates containing kanamycin, colistin sulfate and nalidixic acid.
RNA isolation
Bacterial cultures grown to a defined growth phase were mixed with 0.2 volumes of 5 % phenol in 95 % ethanol 42 and bacteria harvested by centrifugation. Bacterial pellets were frozen in liquid nitrogen and stored at −80°C. RNA from L. monocytogenes was isolated using a modification of guanidinium thiocyanate-phenol-chloroform extraction.43
Northern blotting
For northern blotting, 20 μg of total RNA was separated on a formaldehyde agarose gel prior to blotting as described.43 The Hybond-N membrane (GE Healthcare) was subsequently hybridized with 32P α-dATP-labeled DNA fragments amplified with corresponding primers using Prime-a-Gene DNA labeling system (Promega). Northern blots were developed, and band intensities were measured in the STORM machine (Molecular Dynamics). PCR primer pairs used to generate DNA probes for detection of hly, prfA, inlA and tmRNA are listed in Supplementary Table S1.
Polysome profile
The polysome profiling was essentially performed as previously described,44 with a modification of the lysis method. Listeria monocytogenes strains were grown in BHI shaking culture at 37 or 20°C 160 rpm to an OD600 of 0.5. Bacterial growth was stopped with 100 μg/ml chloramphenicol, cells were harvested by centrifugation of 500 ml culture at 10000 ×g at 4°C, washed with half culture volume ice-cold solution RW (10 mM Tris-Cl pH 7.5, 60 mM KCl, 10 mM MgCl2, 6 mM 2-mercaptoethanol, 1 mM PMSF, 100 μg/ml chloramphenicol) and frozen in liquid nitrogen. Bacterial cells were disrupted using a Freezer/Mill 6870 (Spex Sampleprep, Stanmore, UK). The grindates were suspended in solution RL (10 mM Tris-Cl pH 7.5, 60 mM KCl, 10 mM MgCl2, 6 mM 2-mercaptoethanol, 1 mM PMSF, 100 μg/ml chloramphenicol, 0.2% Triton X100 Reduced (Sigma-Aldrich), 200 U/ml DNase I, RNase free (Roche)). After addition of 0.16% sodium deoxycholate samples were centrifuged at 20000 × g, 4°C, for 1 h. Supernatant was either directly loaded for fractionation in sucrose gradient or frozen in liquid nitrogen to be processed later. The sucrose gradients were prepared using the Gradient Master apparatus (Biocomp, Fredericton, NB, Canada). The amount of lysates, corresponding to 8 units of A260, was loaded on top of centrifugation tubes with formed 10–40 % sucrose gradient in solution R (10 mM Tris-Cl pH 7.5, 60 mM KCl, 10 mM MgCl2). Samples were centrifuged for 3.5 h in SW41Ti rotor (Beckman) at 35000 rpm, 4°C. The ribosomal profiles were generated by UV absorbance A254 measurements of the gradients using an ISCO sucrose gradient fractionator equipped with an UA-6 absorbance detector (Teledyne ISCO, Lincoln, Nebraska, USA).
Primer extension
A method of primer extension using a fluorescently labeled primer was described previously.45 Primer extension reactions were performed using RevertAid Premium reverse transcriptase (Thermo Scientific) according to manufacturer's protocol. Each reaction contained 1 μg of total RNA and 2.4 pmol carboxyfluorescein (6-FAM) labeled primer 23S-FAM 5′-catatcggtgttagtcccg-3′. The primer was allowed to anneal to the template RNA by slowly cooling down the reaction solution from 80°C to 30°C during 1 hour. The rest of reaction components were added to a final volume of 20 μl and primer extension proceeded at 50°C for 1 hour. Reaction products were ethanol precipitated and resolved on 3130xl Genetic Analyzer using a GeneScan 500LIZ Size Standard (Applied Biosystems). Peaks of fluorescent products (corresponding to transcripts of different lengths) were analyzed by GeneMapper 4.0 software (Applied Biosystems).20 From this, the most prominent peak areas corresponding to mature 23S rRNA and immature 23S rRNA harboring a 160 nt 5′-precursor sequence were quantified and the ratio of immature/mature signal was plotted.
SDS-PAGE, Western blotting
Listeria total protein samples for electrophoresis were prepared by mutanolysin lysis,20,46 and analyzed by SDS-PAGE 47 and/or Western blotting on PVDF membrane.48 Different cultures were grown in BHI to an OD600=1. Culture supernatant proteins were concentrated by 6 % trichloracetic acid and sodium deoxycholate precipitation,49 ActA was extracted by boiling the bacteria in 1× Laemmli sample buffer 47 for 10 minutes. Protein samples were separated by a 10% Polyacrylamide gel electrophoresis and either stained with Commassie Brilliant Blue or transferred onto a PVDF membrane using a tank transfer apparatus (Bio-Rad). Development of the membrane followed the protocol of the ECL Prime Western blotting kit (GE Healthcare) using primary antibodies against PrfA, Listeriolysin O or ActA, and HRP-conjugated anti-rabbit secondary antibodies (Bio-Rad). Measurement of luminescence signal was carried out in LAS4000 machine (Fuji).
Cell culture infection assay
Internalization efficiency of L. monocytogenes strains was tested using a Caco-2 gentamicin protection assay.50 Caco-2 cells were grown in DMEM medium supplemented with 10 % fetal calf serum and non-essential amino acids at 37°C in presence of 5 % CO2. Caco-2 were seeded 3 × 104 cells/well on BD BioCoat collagen coated 24-well plates (Corning, Tewksbury, MA, USA). Cells were grown for 24 hours until semi-confluency. Bacterial strains were harvested at OD600=0.5, washed in room temperature with PBS and suspended in complete cell culture medium immediately before the infection. Bacterial strains were added 1.5 × cfu/well and allowed to penetrate for 1 h. After washing twice with warm DMEM medium, Caco-2 cells were incubated for another 1 h with 150 μg/ml gentamicin to kill extracellular bacteria and washed again. Internalized bacteria were released by lysing cells for 30 minutes with ice-cold water. PBS was added to the wells from a 10× stock solution and lysates transferred to Eppendorf tubes. Each tube was vortexed before plating appropriate volumes of bacterial suspension to determine the colony count on tryptic soy agar (TSA) plates.
Viable count dilution test
Single colonies of the EGDe and each mutant strain were inoculated into 10 ml of BHI overnight at 37°C. Cultures grown overnight were diluted to a start OD of 0.02 in fresh BHI. The strains were grown in water bath at 37°C, 25°C and 16°C until an OD600 of 0.5 when the viable cell numbers of the different strains was examined by drop test with indicated dilutions from original culture.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
JJ was supported by Umeå University, the Swedish Research Council grants K2011-56X-15144-08-6 and 621-2012-2451 and an ERC starting grant no 260764 - RNAntibiotics.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene (2006); 367 17–37; PMID:16337753; http://dx.doi.org/ 10.1016/j.gene.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 2. Iost I, Dreyfus M. DEAD-box RNA helicases in Escherichia coli. Nucleic Acids Res (2006); 34:4189-97; PMID:16935881; http://dx.doi.org/ 10.1093/nar/gkl500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lehnik-Habrink M, Pförtner H, Rempeters L, Pietack N, Herzberg C, Stülke J. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex. Mol Microbiol (2010); PMID:20572937; http://dx.doi.org/ 10.1111/j.1365-2958.2010.07264.x [DOI] [PubMed] [Google Scholar]
- 4. Lehnik-Habrink M, Rempeters L, Kovács ÁT, Wrede C, Baierlein C, Krebber H, Kuipers OP, Stülke J. DEAD-Box RNA helicases in Bacillus subtilis have multiple functions and act independently from each other. J Bacteriol (2013); 195:534-44; PMID:23175651; http://dx.doi.org/ 10.1128/jb.01475-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Py B, Higgins CF, Krisch HM, Carpousis AJ. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature (1996); PMID:8610017; 381:169-72, http://dx.doi.org/ 10.1038/381169a0 [DOI] [PubMed] [Google Scholar]
- 6. Bandyra KJ, Bouvier M, Carpousis AJ, Luisi BF. The social fabric of the RNA degradosome. Biochimica et Biophysica acta (2013); PMID:23459248; 1829:514-22, http://dx.doi.org/ 10.1016/j.bbagrm.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehnik-Habrink M, Lewis RJ, Mader U, Stulke J. RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases. Mol Microbiol (2012); 84:1005-1017; PMID:22568516; http://dx.doi.org/ 10.1111/j.1365-2958.2012.08072.x [DOI] [PubMed] [Google Scholar]
- 8. Roux CM, DeMuth JP, Dunman PM. Characterization of components of the Staphylococcus aureus mRNA degradosome holoenzyme-like complex. J Bacteriol (2011); 193:5520-26; PMID:21764917; http://dx.doi.org/ 10.1128/jb.05485-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owttrim GW. RNA helicases: diverse roles in prokaryotic response to abiotic stress. RNA Biol (2013); 10:96-110; PMID:23093803; http://dx.doi.org/ 10.4161/rna.22638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koo JT, Choe J, Moseley SL. HrpA, a DEAH-box RNA helicase, is involved in mRNA processing of a fimbrial operon in Escherichia coli. Mol Microbiol (2004); 52:1813-26; PMID:15186427; http://dx.doi.org/ 10.1111/j.1365-2958.2004.04099.x [DOI] [PubMed] [Google Scholar]
- 11. Salman-Dilgimen A, Hardy PO, Dresser AR, Chaconas G. HrpA, a DEAH-box RNA helicase, is involved in global gene regulation in the Lyme disease spirochete. PloS one (2011); 6, e22168; PMID:21814569; http://dx.doi.org/ 10.1371/journal.pone.0022168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salman-Dilgimen A, Hardy PO, Radolf JD, Caimano MJ, Chaconas G. HrpA, an RNA helicase involved in RNA processing, is required for mouse infectivity and tick transmission of the Lyme disease spirochete. PLoS Pathog (2013); 9, e1003841; PMID:24367266; http://dx.doi.org/ 10.1371/journal.ppat.1003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oun S, Redder P, Didier JP, François P, Corvaglia AR, Buttazzoni E, Giraud C, Girard M, Schrenzel J, Linder P. The CshA DEAD-box RNA helicase is important for quorum sensing control in Staphylococcus aureus. RNA Biol (2013); 10:157-65; PMID:23229022; http://dx.doi.org/ 10.4161/rna.22899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redder P, Linder P. DEAD-box RNA helicases in gram-positive RNA decay. Methods Enzymol (2012); 511:369-83; PMID:22713329; http://dx.doi.org/ 10.1016/b978-0-12-396546-2.00017-6 [DOI] [PubMed] [Google Scholar]
- 15. Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev (2001); 14:584-640; PMID:11432815; http://dx.doi.org/ 10.1128/cmr.14.3.584-640.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nature reviews. Microbiology (2006); 4:423-34; PMID:16710323; http://dx.doi.org/ 10.1038/nrmicro1413 [DOI] [PubMed] [Google Scholar]
- 17. Scortti M, Monzo HJ, Lacharme-Lora L, Lewis DA, Vazquez-Boland JA. The PrfA virulence regulon. Microbes Infect (2007); 9:1196-1207; PMID:17764998; http://dx.doi.org/ 10.1016/j.micinf.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 18. Freitag NE, Port GC, Miner MD. Listeria monocytogenes - from saprophyte to intracellular pathogen. Nature reviews. Microbiology (2009); 7:623-8; PMID:19648949; http://dx.doi.org/ 10.1038/nrmicro2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de las Heras A, Cain RJ, Bieleckal MK, Vazquez-Boland JA. Regulation of Listeria virulence: PrfA master and commander. Curr Opin Microbiol (2011); 14:118-27; PMID:21388862; http://dx.doi.org/Doi 10.1016/J.Mib.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 20. Netterling S, Vaitkevicius K, Nord S, Johansson JA. Listeria monocytogenes RNA helicase essential for growth and ribosomal maturation at low temperatures uses its C terminus for appropriate interaction with the ribosome. J Bacteriol (2012); 194:4377-85; PMID:22707705; http://dx.doi.org/ 10.1128/JB.00348-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markkula A, Lindstrom M, Johansson P, Bjorkroth J, Korkeala H. Roles of four putative DEAD-box RNA helicase genes in growth of Listeria monocytogenes EGD-e under heat, pH, osmotic, ethanol, and oxidative stress conditions. Appl Environ Microbiol (2012); 78:6875-82; PMID:22820328; http://dx.doi.org/ 10.1128/aem.01526-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markkula A, Mattila M, Lindstrom M, Korkeala H. Genes encoding putative DEAD-box RNA helicases in Listeria monocytogenes EGD-e are needed for growth and motility at 3 degrees C. Environ Microbiol (2012); 14:2223-32; PMID:22564273; http://dx.doi.org/ 10.1111/j.1462-2920.2012.02761.x [DOI] [PubMed] [Google Scholar]
- 23. Markkula A, Mattila M, Lindstrom M, Korkeala H. Genes encoding putative DEAD-box RNA helicases in Listeria monocytogenes EGD-e are needed for growth and motility at 3 degrees C. Environ Microbiol (2012); PMID:22564273; http://dx.doi.org/ 10.1111/j.1462-2920.2012.02761.x [DOI] [PubMed] [Google Scholar]
- 24. Jagessar KL, Jain C. Functional and molecular analysis of Escherichia coli strains lacking multiple DEAD-box helicases. RNA (2010); 16:1386-92; PMID:20484467; http://dx.doi.org/ 10.1261/rna.2015610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iost I, Bizebard T, Dreyfus M. Functions of DEAD-box proteins in bacteria: current knowledge and pending questions. Biochimica et Biophysica acta (2013); 1829:866-77; PMID:23415794; http://dx.doi.org/ 10.1016/j.bbagrm.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 26. Charollais J, Pflieger D, Vinh J, Dreyfus M, Iost I. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol Microbiol (2003); 48:1253-65; PMID:12787353 [DOI] [PubMed] [Google Scholar]
- 27. Charollais J, Dreyfus M, Iost I. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res (2004); 32:2751-59; PMID:15148362; http://dx.doi.org/ 10.1093/nar/gkh603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de las Heras A, Cain RJ, Bielecka MK, Vazquez-Boland JA. Regulation of Listeria virulence: PrfA master and commander. Curr Opin Microbiol (2011); 14:118-27; PMID:21388862; http://dx.doi.org/ 10.1016/j.mib.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 29. Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun (1995); 63:3896-903; PMID:7558297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandiani F, Brillard J, Bornard I, Michaud C, Chamot S, Nguyen-the C, Broussolle V. Differential involvement of the five RNA helicases in adaptation of Bacillus cereus ATCC 14579 to low growth temperatures. Appl Environ Microbiol (2010); 76:6692-97; PMID:20709848; http://dx.doi.org/ 10.1128/aem.00782-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandiani F, Chamot S, Brillard J, Carlin F, Nguyen-the C, Broussolle V. Role of the five RNA helicases in the adaptive response of Bacillus cereus ATCC 14579 cells to temperature, pH, and oxidative stresses. Appl Environ Microbiol (2011); 77:5604-09; PMID:21705526; http://dx.doi.org/ 10.1128/aem.02974-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broussolle V, Pandiani F, Haddad N, Michaud C, Carlin F, Nguyen-the C, Brillard J. Insertional mutagenesis reveals genes involved in Bacillus cereus ATCC 14579 growth at low temperature. FEMS Microbiol Lett (2010); 306:177-83; PMID:20370835; http://dx.doi.org/ 10.1111/j.1574-6968.2010.01953.x [DOI] [PubMed] [Google Scholar]
- 33. Lehnik-Habrink M, Rempeters L, Kovács ÁT, Wrede C, Baierlein C, Krebber H, Kuipers OP, Stülke J. DEAD-box RNA helicases in bacillus subtilis have multiple functions and act independently from each other. J Bacteriol (2013); 195:534-44; PMID:23175651; http://dx.doi.org/ 10.1128/Jb.01475-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunger K, Beckering CL, Wiegeshoff F, Graumann PL, Marahiel MA. Cold-induced putative DEAD box RNA helicases CshA and CshB are essential for cold adaptation and interact with cold shock protein B in Bacillus subtilis. J Bacteriol (2006); 188:240-8; PMID:16352840; http://dx.doi.org/ 10.1128/jb.188.1.240-248.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vakulskas CA, Pannuri A, Cortés-Selva D, Zere TR, Ahmer BM, Babitzke P, Romeo T. Global effects of the DEAD-box RNA helicase DeaD (CsdA) on gene expression over a broad range of temperatures. Mol Microbiol (2014); 92:945-58; PMID:24708042; http://dx.doi.org/ 10.1111/mmi.12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Resch A, Vecerek B, Palavra K, Blasi U. Requirement of the CsdA DEAD-box helicase for low temperature riboregulation of rpoS mRNA. RNA Biol (2010); 7:796-802; PMID:21045550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, Repoila F, Buchrieser C, Cossart P, Johansson J. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell (2009); 139:770-9; PMID:19914169; http://dx.doi.org/ 10.1016/j.cell.2009.08.046 [DOI] [PubMed] [Google Scholar]
- 38. Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd edn, (CSHL Press, 2001). [Google Scholar]
- 39. Arnaud M, Chastanet A, Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol (2004); 70:6887-91; PMID:15528558; http://dx.doi.org/ 10.1128/AEM.70.11.6887-6891.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monk IR, Gahan CG, Hill C. Tools for functional postgenomic analysis of Listeria monocytogenes. Appl Environ Microbiol (2008); 74:3921-34; PMID:18441118; http://dx.doi.org/ 10.1128/AEM.00314-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flamm RK, Hinrichs DJ, Thomashow MF. Introduction of pAM beta 1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect Immun (1984); 44:157-61; PMID:6323313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Udekwu KI, Darfeuille F, Vogel J, Reimegård J, Holmqvist E, Wagner EG. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev (2005); 19:2355-66; PMID:16204185; http://dx.doi.org/ 10.1101/gad.354405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, Loh E, Gripenland J, Tiensuu T, Vaitkevicius K, et al. . The Listeria transcriptional landscape from saprophytism to virulence. Nature (2009); 459:950-6; PMID:19448609; http://dx.doi.org/ 10.1038/nature08080 [DOI] [PubMed] [Google Scholar]
- 44. Gould H, Herbert BN, Loviny T. Polysomes from bacillus subtilis and bacillus thuringiensis. Nature (1969); 223:855-7; PMID:4979045 [DOI] [PubMed] [Google Scholar]
- 45. Lloyd AL, Marshall BJ, Mee BJ. Identifying cloned Helicobacter pylori promoters by primer extension using a FAM-labelled primer and GeneScan analysis. J Microbiol Methods (2005); 60:291-8; PMID:15649531; http://dx.doi.org/ 10.1016/j.mimet.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 46. Fliss I, Emond E, Simard RE, Pandian S. A rapid and efficient method of lysis of Listeria and other gram-positive bacteria using mutanolysin. BioTech (1991); 11, 453:456-7. [PubMed] [Google Scholar]
- 47. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (1970); 227:680-5; PMID:5432063 [DOI] [PubMed] [Google Scholar]
- 48. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A (1979); 76:4350-54; PMID:388439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem (1976); 70:241-50; PMID:1259145 [DOI] [PubMed] [Google Scholar]
- 50. Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun (1987); 55:2822-29; PMID:3117693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bethesda Research Laboratories BRL pUC host: E. coli DH5α competent cells. Focus (1986); 8:9. [Google Scholar]
- 52. Simon R, Priefer U, Puhler A. A broad host range mobilization system for In Vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotech (1983); 1:784-91. [Google Scholar]
- 53. Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, et al. . Comparative genomics of Listeria species. Science (2001); 294:849-52; PMID:11679669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


