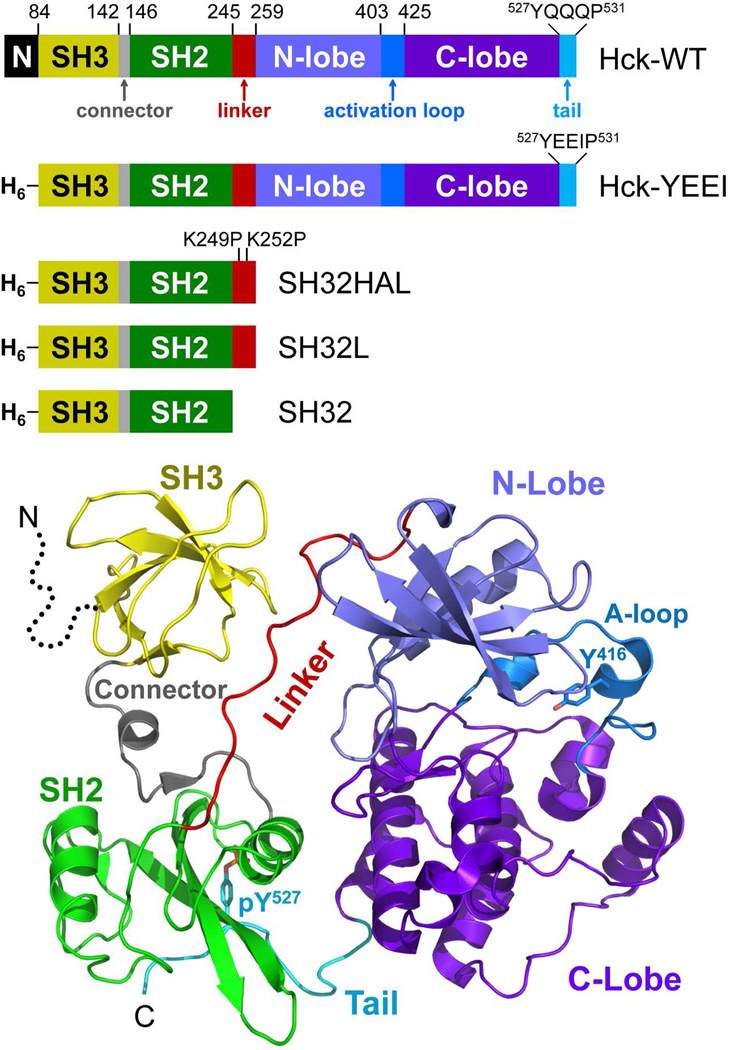
Figure 1.
Structure of Hck and diagram of the recombinant proteins used in this study. Top: Hck consists of an N-terminal unique domain (N), regulatory SH3 and SH2 domains separated by a short connector, an SH2-kinase linker, and a bi-lobed kinase domain with small N-terminal and large C-terminal lobes. The activation loop contains the autophosphorylation site (Tyr416) while the wild-type regulatory tail contains the regulatory phosphotyrosine (pTyr527) followed by the sequence QQQP. In recombinant Hck-YEEI, the N-terminal region is replaced with a His-tag (H6) and the C-terminal tail sequence is modified to YEEIP. Smaller regulatory domain constructs include the SH3-SH2 unit plus a modified, high-affinity linker in which the two linker lysines shown are replaced with prolines (SH32HAL), the SH3-SH2 unit plus the wild-type linker (SH32L), and the SH3-SH2 unit without the linker (SH32). Bottom: Crystal structure of downregulated Hck-YEEI, with key structural elements colored as per the diagram at the top (PDB: 1QCF)18. The N-terminal unique domain is not present in the structure (dotted line).