Abstract
The second messenger cyclic di-GMP (c-di-GMP) regulates numerous phenotypes in response to environmental stimuli to enable bacteria to transition between different lifestyles. Here we discuss our recent findings that the human pathogen Vibrio cholerae recognizes 2 host-specific signals, bile and bicarbonate, to regulate intracellular c-di-GMP. We have demonstrated that bile acids increase intracellular c-di-GMP to promote biofilm formation. We have also shown that this bile-mediated increase of intracellular c-di-GMP is negated by bicarbonate, and that this interaction is dependent on pH, suggesting that V. cholerae uses these 2 environmental cues to sense and adapt to its relative location in the small intestine. Increased intracellular c-di-GMP by bile is attributed to increased c-di-GMP synthesis by 3 diguanylate cyclases (DGCs) and decreased expression of one phosphodiesterase (PDE) in the presence of bile. The molecular mechanisms by which bile controls the activity of the 3 DGCs and the regulators of bile-mediated transcriptional repression of the PDE are not yet known. Moreover, the impact of varying concentrations of bile and bicarbonate at different locations within the small intestine and the response of V. cholerae to these cues remains unclear. The native microbiome and pharmaceuticals, such as omeprazole, can impact bile and pH within the small intestine, suggesting these are potential unappreciated factors that may alter V. cholerae pathogenesis.
Keywords: bicarbonate, bile, cyclic di-GMP, GGDEF, HD-GYP, Vibrio cholerae
Abbreviations
- c-di-GMP
3′, 5′-cyclic diguanylic acid
- DGC
diguanylate cyclase
- PDE
phosphodiesterase
- LC-MS/MS
liquid chromatography coupled with tandem mass spectrometry
- WT
wild type
- BV
bovine bile
- SHB
synthetic human bile
Discussion
To survive and thrive in diverse and perpetually changing environments, bacteria employ numerous strategies to sense and adapt to their surroundings. One such system is the second messenger 3′,5′-cyclic diguanylic acid (c-di-GMP) signaling network. Similar to other cyclic nucleotide second messenger systems such as cGMP or cAMP, the intracellular bacteria-specific second messenger c-di-GMP is produced in response to external signals and enables rapid signal transduction to facilitate regulatory responses to changing environmental conditions. First described in 1987 by Benziman et al.,1 c-di-GMP has since been shown to be utilized by over 85% of known bacteria.2 Synthesis of c-di-GMP is performed by diguanylate cyclases (DGC) containing conserved GGDEF domains and hydrolyzed by c-di-GMP specific phosphodiesterases (PDE) containing conserved EAL or HD-GYP domains. C-di-GMP has been shown to regulate numerous phenotypes in bacteria, including motility, biofilm formation, virulence, and lifecycle progression (for a comprehensive review of c-di-GMP regulation, please refer to3).
Bacteria use c-di-GMP signaling to mediate the transition between different lifestyles. High intracellular c-di-GMP promotes a sessile lifestyle where biofilm formation is preferred whereas low intracellular c-di-GMP promotes a motile, virulent lifestyle. To optimize lifestyle to different growth environments, enzymes involved in c-di-GMP turnover sense specific environmental cues to modulate intracellular c-di-GMP. There are several examples of environmental cues altering c-di-GMP turnover in various bacteria. It has been shown that amino acids alter intracellular c-di-GMP concentrations of Pseudomonas aeruginosa.4 In Escherichia coli, zinc inhibits the c-di-GMP synthesis of the DGC YdeH5 while oxygen modulates the c-di-GMP turnover of a DGC/PDE complex.6 And in photosynthetic cyanobacteria, light conditions can alter intracellular c-di-GMP concentrations7; for example, in Rhodopseudomonas palustris blue light can indirectly affect the c-di-GMP hydrolysis of the PDE PapA.8
One bacterial model system used to study c-di-GMP signaling is Vibrio cholerae. In marine environments, V. cholerae preferentially forms biofilms on chitinous surfaces.9-11 However, upon ingestion into a human host V. cholerae becomes the causative agent of the human diarrheal disease cholera. C-di-GMP regulates numerous phenotypes important for V. cholerae virulence. Specifically, c-di-GMP increases biofilm formation in V. cholerae12 while inhibiting motility and virulence regulators.13-15 Furthermore, it has been shown that c-di-GMP turnover enzymes are differentially regulated in the human host.15 Based on these phenotypes, it has been proposed that c-di-GMP is elevated in marine environments to promote biofilm formation and repressed in the host to promote motility and virulence. Therefore, we recently published a study examining the role of human specific signals in the regulation of c-di-GMP in V. cholerae.16
One human-specific cue that bacteria encounter in the human digestive tract is bile. Bile, composed of bile acids, salts, cholesterol, phospholipids, and the pigment biliverdin, is a heterogeneous mixture produced in the liver and secreted into the proximal small intestine (Fig. 1).17 The purpose of bile acids is to solubilize fats in order to aid digestion. Bile has been shown to have potent antimicrobial activities in vitro, leading to the conclusion that one role of bile is to control microbial flora in the intestine. In bacteria, bile can cause membrane damage, oxidative stress, alter protein conformation, and disrupt iron and calcium homeostasis.17
Figure 1.
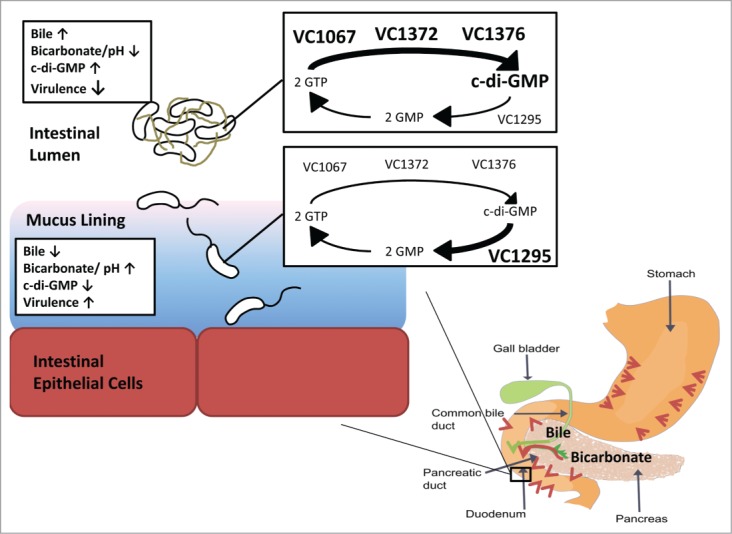
Proposed model of the role of bile and bicarbonate in c-di-GMP regulation and V. cholerae infection. In the digestive tract, bile (green arrow) is secreted from the gall bladder into the duodenum, while bicarbonate (red arrows) is secreted from the stomach, pancreas, and intestinal epithelium. In the lumen where bile is most concentrated, the intracellular c-di-GMP of V. cholerae is elevated by increased c-di-GMP synthesis of the 3 DGCs, VC1067, VC1372, and VC1376, and reduced transcription of one PDE, VC1295. In contrast, bicarbonate is more concentrated in the mucus-bicarbonate barrier lining the intestinal epithelium leading to increased pH. This suppresses the activities of the 3 DGCs and increases expression of VC1295, resulting in decreased intracellular c-di-GMP of V. cholerae adjacent to intestinal epithelial cells.
The relationship between bacteria and bile is complex. Bacteria induce numerous physiological changes to adapt to the stress induced by bile. While the lipopolysacharide grants some protection against bile in gram-negative bacteria, efflux pumps are important for bile resistance.17 Additionally, it has been demonstrated in Bifidobacteria that bile induces changes in membrane properties and lipid composition.18 Outer-membrane porin composition can also influence bile tolerance, and bacteria can alter their OM porin composition in response to bile.19-21 Bacteria also express bile salt hydrolases which deconjugate host bile acids; numerous bacteria associated with the native host microbiota express these enzymes that lead to secondary bile acid formation and produce significant changes in the host bile acid pool.22 Not surprisingly, disruptions in the host microbiota can alter bile acid composition.23
We sought to examine if bile regulates c-di-GMP in V. cholerae. Based on the prevailing model that c-di-GMP levels are suppressed in the host, we hypothesized that bile would reduce intracellular c-di-GMP in V. cholerae. To our surprise, we found that either bovine bile (BV) or synthetic human bile (SHB) increased intracellular c-di-GMP 3–10 fold in V. cholerae. As SHB consists solely of 6 purified bile acids, we concluded that the bile acid component of bile increases the intracellular c-di-GMP concentration of V. cholerae. It is not known if other components of bile can also increase c-di-GMP, and likewise if specific bile acids are able to increase intracellular c-di-GMP, or if this effect is additive and non-specific to individual bile acids. This observation that bile acids increase intracellular c-di-GMP in V. cholerae suggests that c-di-GMP regulation of V. cholerae in the human host is more complex than previously appreciated, and that phenotypes controlled by high c-di-GMP may have a selective advantage within the small intestine.
Increased c-di-GMP in V. cholerae represses virulence factor expression.14,15 Why then would bile, an environmental cue signaling habitation in the small intestine, increase c-di-GMP? Bile-induced c-di-GMP could serve numerous functions during V. cholerae pathogenesis. We found that bile-induced c-di-GMP production stimulated increased biofilm formation on a surface and autoaggregation in liquid, which could protect cells from stresses associated with the small intestine such as bile, acid stress, antimicrobial peptides, or host IgA. In support of this idea, rice water stools from cholera patients contain V. cholerae aggregates, suggesting autoaggregation occurs in vivo during infection.24 Alternatively, high c-di-GMP could reduce the expression of virulence factors such as cholera toxin until the bacteria are near the intestinal epithelium where maximum impact can be achieved. This model of localized virulence factor expression in the small intestine has been previously proposed for both V. cholerae and Salmonella enterica.25-27
We therefore postulated that other host-specific signals located more proximal to the duodenal epithelium would decrease intracellular c-di-GMP in V. cholerae, and our prime candidate for this cue was bicarbonate. Bicarbonate is secreted into the small intestine from the stomach, the pancreas (from the sphincter of Oddi), and directly by intestinal epithelial cells to form the mucus-bicarbonate barrier, all of which counteract the detrimental effects of stomach acid and modulate intestinal pH (Fig. 1).28,29 Moreover, bicarbonate is known to positively activate V. cholerae virulence gene expression.27 Bicarbonate alone had no effect on intracellular c-di-GMP concentrations in V. cholerae; however, co-administration of bicarbonate with SHB quenched the induction of c-di-GMP caused by bile acids.
We do not yet know the mechanism by which bicarbonate overrides bile induction of c-di-GMP in V. cholerae, but we observed a similar inhibition with Tris buffer at pH 8.0 suggesting this effect is due to changes in pH. We consider 2 possibilities to explain this finding. One, bicarbonate and/or pH changes may alter the chemistry of the bile acids, which negates the c-di-GMP response of V. cholerae. In support of this hypothesis, it has been proposed that conjugated bile acids can enter the cell and become deprotonated in the alkaline intracellular environment, thus reducing intracellular pH.30 The more alkaline bicarbonate-supplemented growth media would promote bile acid deprotonation prior to entering the cell, preventing intracellular acidification. Similarly, pH driven changes in bile structure could render it unable to bind receptors in V. cholerae. Alternatively, bicarbonate and/or pH may alter the activity of bile-responsive factors in the cell, rendering them insensitive to bile. In support of this model, bicarbonate regulates virulence gene expression through the transcriptional regulator ToxT27; thus it is possible that bicarbonate regulates the transcription or post-transcriptional activities of DGCs or PDEs.
This seemingly inverse regulation of c-di-GMP in response to bile and bicarbonate leads us to propose the following model. After ingestion into a human host, V. cholerae enters the small intestine where the bacteria encounter high concentrations of bile acids and low pH from stomach acids in the lumen, leading to increased intracellular c-di-GMP in this microenvironment (Fig. 1). Elevated c-di-GMP promotes biofilm formation, which could grant the cell protection against various stresses associated with the intestinal lumen. If V. cholerae is unable to adhere to the intestinal epithelial cells, it would emerge from the host in a biofilm state, potentially being more adapted for environmental survival. When V. cholerae penetrate the mucus lining of the small intestine, the bile acid concentration decreases and the pH increases, leading to decreased intracellular c-di-GMP. Decreased c-di-GMP promotes motility and virulence factor expression, which could allow the cell to move close to the intestinal epithelial cells and enable localized cholera toxin secretion. This coordinated control of intracellular c-di-GMP in V. cholerae by 2 distinct host-specific signals enables localized control of virulence in the human host.
To clarify the mechanism by which bile and bicarbonate inversely control c-di-GMP in V. cholerae, we identified the intracellular effectors leading to high c-di-GMP in the presence of bile. This was not an easy task as V. cholerae encodes 60 proteins that are predicted to synthesize or degrade c-di-GMP, each of which can be controlled at the levels of expression or enzymatic activity. Therefore, we measured the expression of each of these 60 genes using either gfp or luciferase transcriptional fusion reporters in the presence and absence of bile. Only one gene, the PDE VC1295 containing a HD-GYP domain, showed significant bile-mediated regulation as it was repressed by bile acid addition. As this protein is predicted to degrade c-di-GMP, repression of this gene would increase c-di-GMP concentrations, consistent with our observations.
Differential gene expression in the presence of bile has been previously reported in V. cholerae, and numerous mechanisms to modulate transcription in the presence of bile have been described. The regulator BreR has been shown to bind the promoter region of breAB efflux system and breR to repress transcription unless bile is present.31,32 Additionally, bile induces disulfide bonds in the trans-membrane transcriptional regulator TcpP, which subsequently regulates virulence gene expression.33 And finally, the activity of the transcriptional regulator ToxR differentially regulates the expression of cholera toxin and outer membrane proteins in the presence of bile.21,34,35 It is possible that one or more of these transcriptional regulators is responsible for regulating the expression of VC1295 in the presence of bile. Consistent with this hypothesis, there are sites upstream of the VC1295 gene that resemble the known ToxR binding site motif.36 We hypothesize that ToxR represses the expression of VC1295 in the presence of bile acids, and this repression is alleviated when bile acids are absent. Further studies are necessary to determine what transcriptional regulators are driving changes in VC1295 expression.
Examining the activity of each of the 60 DGC and PDE enzymes in the presence and absence of bile acids to determine bile-mediated regulation required a bit more manipulation. We reasoned that a c-di-GMP inducible luminescence transcriptional fusion that we had previously identified would function as an in vivo readout of c-di-GMP levels.37 We constructed expression vectors of each individual DGC encoding gene where transcription was controlled by the inducible Ptac promoter and translation was driven by a conserved ribosome binding site (Fig. 2A). V. cholerae strains harboring both the DGC expression vector and the lux reporter were grown in the presence and absence of bile acids, and we determined the differences in luminescence. We found 3 DGCs, VC1067, VC1372, and VC1376, that increased luminescence upon ectopic expression in V. cholerae grown in the presence of bile acids, indicating that these enzymes increased c-di-GMP synthesis (Fig. 1). This result was confirmed by quantifying the intracellular c-di-GMP concentrations of the V. cholerae DGC expression strains in the presence and absence of bile acids.
Figure 2.
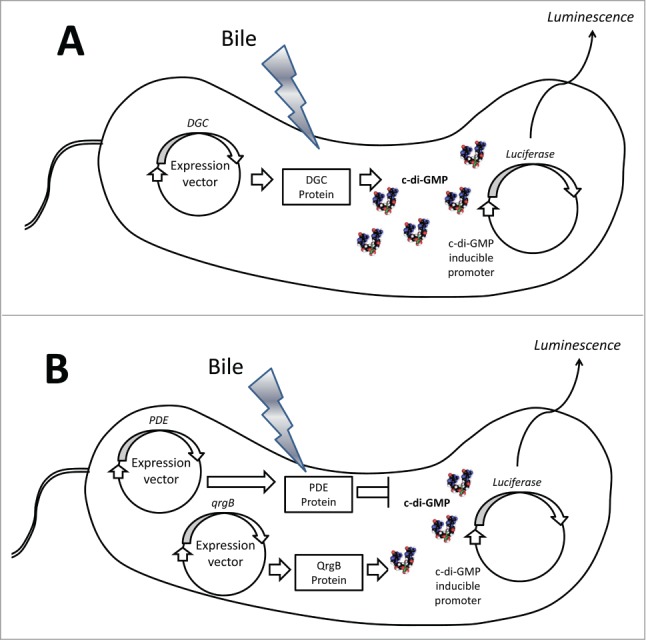
Representation of the high-throughput screen to quantify relative DGC and PDE activity. (A) To determine DGC activity in response to bile, individual DGCs are expressed from an IPTG-inducible vector in V. cholerae. Relative c-di-GMP synthesis is determined using a separate reporter vector containing the lux operon under the control of a c-di-GMP inducible promoter (6:C9-lux). Luminescence from each strain was measured in the presence and absence of bile. (B) To determine relative PDE activity, the DGC qrgB is expressed alongside individual PDEs from IPTG-inducible vectors. The same c-di-GMP lux reporter vector is measured in the presence and absence of bile to determine relative c-di-GMP hydrolysis.
We also wanted to examine the effect of bile acids on the c-di-GMP hydrolytic activity of the V. cholerae PDEs. However, the baseline luminescence of our reporter vector was too low to resolve decreases as a result of PDE over-expression. To overcome this limitation, we introduced a third ectopic expression vector containing the DGC qrgB from V. harveyi. QrgB constitutively produces c-di-GMP, resulting in increased baseline c-di-GMP and luminescence (Fig. 2B). We were then able to express each V. cholerae PDE and measure the reduction of luminescence. None of the PDE expression strains demonstrated increased luminescence in the presence of bile acids, suggesting that the hydrolysis activity of the PDEs was not decreased by bile. These assays function as a robust, semi-high throughput system to measure the activity of every DGC and PDE in different environmental conditions, and similar approaches can be developed for other bacteria.
The mechanism by which these 3 DGCs increase c-di-GMP synthesis in the presence of bile is not yet known. Each of the bile-responsive DGCs is predicted to contain trans-membrane domains in the N-terminus of the protein. As bile acids are known to interact with and disrupt the cellular membrane of bacteria, we hypothesize that it the bile-dependent change in c-di-GMP synthesis is dependent on these trans-membrane domains. It is possible that bile-induced alterations in the physicochemical properties of the inner membrane cause changes in DGC activity, either by direct perturbations of the membrane by bile acids or indirectly by causing V. cholerae to modify the lipid topology of the membrane in response to the stress of bile acids. Alternatively, the bile-responsive activity of these DGCs could be dependent on a separate effector protein capable of sensing bile acids and interacting with these enzymes. This would be consistent with other studies demonstrating that c-di-GMP turnover enzymes can operate in heterogeneous multi-enzyme complexes.6,8 We found that the bile-dependent c-di-GMP synthesis of VC1372 was maintained in E. coli, suggesting that if a separate effector is involved it is conserved in this related enteric bacteria.
To verify that these c-di-GMP turnover enzymes are responsible for increasing the intracellular c-di-GMP concentration of V. cholerae grown in the presence of bile acids, we generated deletion mutants of each of the 3 bile-responsive DGCs as well as the PDE VC1295. Additionally, we deleted these genes in combination to determine if these 4 enzymes completely account for the bile-mediated increased intracellular c-di-GMP in V. cholerae. These strains were grown in the presence and absence of SHB, and intracellular c-di-GMP was quantified using LC-MS/MS. Individual mutations of the bile-responsive DGCs had only a modest effect on intracellular c-di-GMP when the strains were grown in bile. Only the quadruple DGC/PDE mutant had no significant difference in intracellular c-di-GMP when grown in LB and LB with SHB, showing that these 4 enzymes together are responsible for increasing c-di-GMP in the presence of bile. While the WT strain of V. cholerae induces biofilm formation on surfaces and autoaggregation in liquid in response to bile, this quadruple mutant no longer increased biofilm in the presence of bile showing this response is c-di-GMP dependent.
The fact that 4 c-di-GMP turnover enzymes are involved in increasing intracellular c-di-GMP and subsequently increasing biofilm formation in the presence of bile is evidence that c-di-GMP signaling systems can involve significant redundancy. This high level of redundancy suggests that this response to bile is important for the fitness of V. cholerae, either in the human host or in natural reservoirs. It is possible that the seemingly redundant response of these 4 c-di-GMP turnover enzymes to bile is subject to signaling specificity, where c-di-GMP turnover of individual enzymes differentially regulates specific phenotypes; this phenomenon of c-di-GMP signaling specificity has been previously described in V. cholerae.38 Further investigation is necessary to determine what impact each individual enzyme has on c-di-GMP associated phenotypes in the presence of bile.
Additionally, c-di-GMP signaling is widely utilized among bacteria2; thus it is reasonable to speculate that other intestinal bacteria also modify c-di-GMP in response to bile acids and bicarbonate. If indeed the ratio of bile and bicarbonate function as cues indicating relative location within the small intestine, we would hypothesize that other enteric pathogens, especially those that colonize the small intestine such as pathogenic Escherichia coli, Yersinia, and Salmonella,39 would similarly utilize this information to modify c-di-GMP signaling and virulence. The N-terminal domains of the bile-responsive DGCs that we identified are not conserved outside of the Vibrio family. Therefore, if other enteric pathogens respond similarly to bile and bicarbonate they utilize different mechanisms.
Our finding that bile acids and bicarbonate modify c-di-GMP signaling in V. cholerae have important implications as certain medical conditions such as malnourishment, obstructions in enterohepatic circulation, or defective bile re-absorption can alter intestinal bile,17 which could shift c-di-GMP levels in the intestinal microbiota and thus the community structure. Alternatively, modified bile levels in the host could lead to altered intracellular c-di-GMP of intestinal pathogens, leading to increased probability or severity of disease. Changes in the host microbiota are associated with altered bile acid profiles, providing a potential unexplored molecular interaction between the microbiome and V. cholerae.23 Furthermore, numerous drugs alter bile composition and abundance; these drugs could also change c-di-GMP levels of intestinal bacteria.40 In addition, the role of pH regulating c-di-GMP in V. cholerae could have implications for the effects of medications effecting intestinal pH on susceptibility to V. cholerae infections. For example, proton pump inhibitors such as omeprazole suppress stomach acid secretion and raise intestinal pH; these drugs could affect the intracellular c-di-GMP of V. cholerae, making an individual more susceptible to disease.41 A better understanding of the interaction of V. cholerae with bile and bicarbonate in vivo is needed to decipher the implications of these factors on V. cholerae infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the NIH grant U19AI090872 and R21AI105499 and the MSU Foundation. We also would like to acknowledge the Rudolph Hugh Fellowship, the Russell B. DuVall Scholarship, and the Marvin Hensley Fellowship to B.J.K., and assistance from the MSU mass spectrometry facility to quantify c-di-GMP.
References
- 1. Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinbergerohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, et al. . Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987; 325:279-81; PMID:18990795; http://dx.doi.org/ 10.1038/325279a0 [DOI] [PubMed] [Google Scholar]
- 2. Seshasayee ASN, Fraser GM, Luscombe NM. Comparative genomics of cyclic-di-GMP signaling in bacteria: post-translational regulation and catalytic activity. Nucleic Acids Res 2010; 38:5970-81; PMID:20483912; http://dx.doi.org/ 10.1093/nar/gkq382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the First 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 2013; 77:1-52; PMID:23471616; http://dx.doi.org/ 10.1128/MMBR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernier SP, Ha DG, Khan W, Merritt JH, O’Toole GA. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol 2011; 162:680-8; PMID:21554951; http://dx.doi.org/ 10.1016/j.resmic.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zähringer F, Lacanna E, Jenal U, Schirmer T, Boehm A. Structure and signaling mechanism of a zinc-sensory diguanylate cyclase. Structure 2013; 21:1149-57; PMID:23769666; 10.1016/j.str.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 6. Tuckerman JR, Gonzalez G, Sousa EHS, Wan XH, Saito JA, Alam M, Gilles-Gonzalez MA. An oxygen-sensing diguanylate cyclase and phosphodiesterase couple for c-di-GMP control. Biochemistry 2009; 48:9764-74; PMID:19764732; http://dx.doi.org/ 10.1021/bi901409g [DOI] [PubMed] [Google Scholar]
- 7. Agostoni M, Koestler BJ, Waters CM, Williams BL, Montgomery BL. Occurrence of cyclic di-GMP-modulating output domains in cyanobacteria: an illuminating perspective. MBio 2013; 4:pii: e00451-13; PMID:23943760; http://dx.doi.org/ 10.1128/mBio.00451-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanazawa T, Ren S, Maekawa M, Hasegawa K, Arisaka F, Hyodo M, Hayakawa Y, Ohta H, Masuda S. Biochemical and physiological characterization of a BLUF protein-EAL protein complex involved in blue light-dependent degradation of cyclic diguanylate in the purple bacterium Rhodopseudomonas palustris. Biochemistry 2010; 49:10647-55; PMID:21082778; http://dx.doi.org/ 10.1021/bi101448t [DOI] [PubMed] [Google Scholar]
- 9. Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 1983; 45:275-83; PMID:6337551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR. Attachment of Vibrio cholerae serogroup-O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol 1990; 56:1977-80; PMID:2383016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halpern M, Broza YB, Mittler S, Arakawa E, Broza M. Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb Ecol 2004; 47:341-9; PMID:14681736; http://dx.doi.org/ 10.1007/s00248-003-2007-6 [DOI] [PubMed] [Google Scholar]
- 12. Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 2004; 53:857-69; PMID:15255898; http://dx.doi.org/ 10.1111/j.1365-2958.2004.04155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srivastava D, Hsieh M-L, Khataokar A, Neiditch MB, Waters CM. Cyclic di-GMP inhibits Vibrio cholerae motility by repressing induction of transcription and inducing extracellular polysaccharide production. Mol Microbiol 2013; 90:1262-76; PMID:24134710; http://dx.doi.org/ 10.1111/mmi.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 2005; 73:5873-82; PMID:16113306; http://dx.doi.org/ 10.1128/IAI.73.9.5873-5882.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamayo R, Schild S, Pratt JT, Camili A. Role of cyclic di-GMP during El tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic di-GMP phosphodiesterase CdpA. Infect Immun 2008; 76:1617-27; PMID:18227161; http://dx.doi.org/ 10.1128/IAI.01337-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koestler BJ, Waters CM. Bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in vibrio cholerae. Infect Immun 2014; 82:3002-14; PMID:24799624; http://dx.doi.org/ 10.1128/IAI.01664-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 2005; 29:625-51; PMID:16102595; http://dx.doi.org/ 10.1016/j.femsre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 18. Gómez Zavaglia A, Kociubinski G, Pérez P, Disalvo E, De Antoni G. Effect of bile on the lipid composition and surface properties of bifidobacteria. J Appl Microbiol 2002; 93:794-9; PMID:12392525; http://dx.doi.org/ 10.1046/j.1365-2672.2002.01747.x [DOI] [PubMed] [Google Scholar]
- 19. Provenzano D, Klose KE. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci U S A 2000; 97:10220-4; PMID:10944196; http://dx.doi.org/ 10.1073/pnas.170219997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wibbenmeyer JA, Provenzano D, Landry CF, Klose KE, Delcour AH. Vibrio cholerae OmpU and OmpT porins are differentially affected by bile. Infect Immun 2002; 70:121-6; PMID:11748172; http://dx.doi.org/ 10.1128/IAI.70.1.121-126.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mey AR, Craig SA, Payne SM. Effects of amino acid supplementation on porin expression and ToxR levels in vibrio cholerae. Infect Immun 2012; 80:518-28; PMID:22144480; http://dx.doi.org/ 10.1128/IAI.05851-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006; 47:241-59; PMID:16299351; http://dx.doi.org/ 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 23. Berr F, Kullak-Ublick GA, Paumgartner G, Munzing W, Hylemon PB. 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology 1996; 111:1611-20; PMID:8942741; http://dx.doi.org/ 10.1016/S0016-5085(96)70024-0 [DOI] [PubMed] [Google Scholar]
- 24. Faruque SM, Biswas K, Udden SMN, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A 2006; 103:6350-5; PMID:16601099; http://dx.doi.org/ 10.1073/pnas.0601277103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prouty AM, Gunn JS. Salmonella enterica serovar typhimurium invasion is repressed in the presence of bile. Infect Immun 2000; 68:6763-9; PMID:11083793; http://dx.doi.org/ 10.1128/IAI.68.12.6763-6769.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krukonis ES, DiRita VJ. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Curr Opin Microbiol 2003; 6:186-90; PMID:12732310; http://dx.doi.org/ 10.1016/S1369-5274(03)00032-8 [DOI] [PubMed] [Google Scholar]
- 27. Abuaita BH, Withey JH. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 2009; 77:4111-20; PMID:19564378; http://dx.doi.org/ 10.1128/IAI.00409-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hogan DL, Ainsworth MA, Isenberg JI. Gastroduodenal bicarbonate secretion. Aliment Pharm Therap 1994; 8:475-88; http://dx.doi.org/ 10.1111/j.1365-2036.1994.tb00319.x [DOI] [PubMed] [Google Scholar]
- 29. Seidler U, Sjöblom M. Gastroduodenal bicarbonate secretion. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. San Diego: Elsevier, 2012:1311-39. [Google Scholar]
- 30. Desmet I, Vanhoorde L, Woestyne MV, Christiaens H, Verstraete W. Significance of bile-salt hydrolytic activities of lactobacilli. J Appl Bacteriol 1995; 79:292-301; PMID:7592123; http://dx.doi.org/ 10.1111/j.1365-2672.1995.tb03140.x [DOI] [PubMed] [Google Scholar]
- 31. Cerda-Maira FA, Ringelberg CS, Taylor RK. The bile response repressor BreR regulates expression of the vibrio cholerae breAB Efflux system operon. J Bacteriol 2008; 190:7441-52; PMID:18776020; http://dx.doi.org/ 10.1128/JB.00584-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerda-Maira FA, Kovacikova G, Jude BA, Skorupski K, Taylor RK. Characterization of BreR interaction with the bile response promoters breAB and breR in vibrio cholerae. J Bacteriol 2013; 195:307-17; PMID:23144245; http://dx.doi.org/ 10.1128/JB.02008-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang MH, Liu Z, Hughes C, Stern AM, Wang H, Zhong Z, Kan B, Fenical W, Zhu J. Bile salt-induced intermolecular disulfide bond formation activates Vibrio cholerae virulence. Proc Natl Acad Sci U S A 2013; 110:2348-53; PMID:23341592; http://dx.doi.org/ 10.1073/pnas.1218039110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hung DT, Mekalanos JJ. Bile acids induce cholera toxin expression in Vibrio cholerae in a ToxT-independent manner. Proc Natl Acad Sci U S A 2005; 102:3028-33; PMID:15699331; http://dx.doi.org/ 10.1073/pnas.0409559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Provenzano D, Schuhmacher DA, Barker JL, Klose KE. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun 2000; 68:1491-7; PMID:10678965; http://dx.doi.org/ 10.1128/IAI.68.3.1491-1497.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goss TJ, Morgan SJ, French EL, Krukonis ES. ToxR recognizes a direct repeat element in the toxT, ompU, ompT and ctxA promoters of Vibrio cholerae to regulate transcription. Infect Immun 2013; 81:884-95; PMID:23297386; http://dx.doi.org/ 10.1128/IAI.00889-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol 2011; 193:6331-41; PMID:21926235; http://dx.doi.org/ 10.1128/JB.05167-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massie JP, Reynolds EL, Koestler BJ, Cong J-P, Agostoni M, Waters CM. Quantification of high-specificity cyclic diguanylate signaling. Proc Natl Acad Sci U S A 2012; 109:12746-51; PMID:22802636; http://dx.doi.org/ 10.1073/pnas.1115663109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garner CD, Antonopoulos DA, Wagner B, Duhamel GE, Keresztes I, Ross DA, Young VB, Altier C. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a salmonella enterica serovar typhimurium murine model of infection. Infect Immun 2009; 77:2691-702; PMID:19433544; http://dx.doi.org/ 10.1128/IAI.01570-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okolicsanyi L, Lirussi F, Strazzabosco M, Jemmolo RM, Orlando R, Nassuato G, Muraca M, Crepaldi G. The effect of drugs on bile-flow and composition–an overview. Drugs 1986; 31:430-48; PMID:2872047; http://dx.doi.org/ 10.2165/00003495-198631050-00003 [DOI] [PubMed] [Google Scholar]
- 41. Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology 2010; 139:1115-27; PMID:20727892; http://dx.doi.org/ 10.1053/j.gastro.2010.08.023 [DOI] [PubMed] [Google Scholar]


