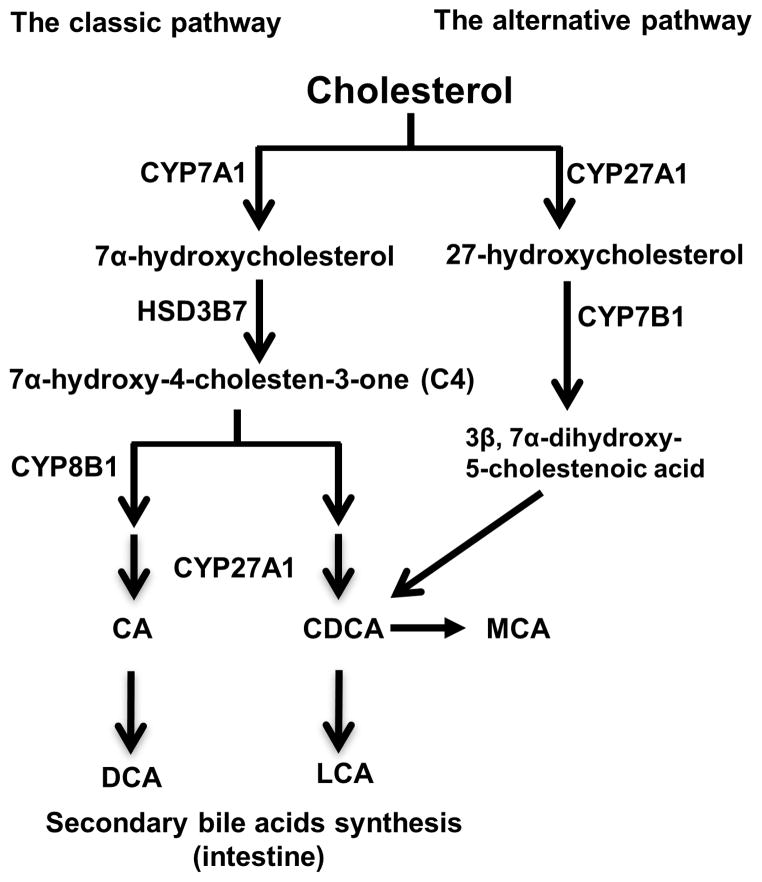
Figure 1. Bile acid synthetic pathways and bile acid structure.
Cholesterol is the common precursor for bile acid synthesis via two major bile acid biosynthetic pathways. In the classic pathway, the rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1) in the endoplasmic reticulum converts cholesterol into 7α-hydroxycholesterol. The 3β-hydroxysteroid dehydrogenase (3βHSD, HSD3B7) converts 7α-hydroxycholesterol to 7α-hydroxy-4 cholesten-3-one (C4). C4 can be converted to cholic acid (CA) which requires the sterol 12α-hydroxylase (CYP8B1). Without 12α-hydroxylation by CYP8B1, C4 is eventually converted to chenodeoxycholic acid (CDCA). In the classic pathway, the mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes the steroid side-chain oxidation in both CA and CDCA synthesis. In the alternative pathway, CYP27A1 catalyzes the first step to convert cholesterol to 27-hydroxycholesterol. Oxysterol 7α-hydroxylase (CYP7B1) catalyzes hydroxylation of 27-hydroxycholesterol to 3β, 7α-dihydroxy-5-cholestenoic acid, which eventually is converted to CDCA. In the large intestine, bacterial 7α-dehydroxylase removes a hydroxyl group from C-7 and converts CA to deoxycholic acid (DCA) and CDCA to lithocholic acid (LCA). In mouse liver, most of CDCA is converted to α- and β-muricholic acid (MCA).