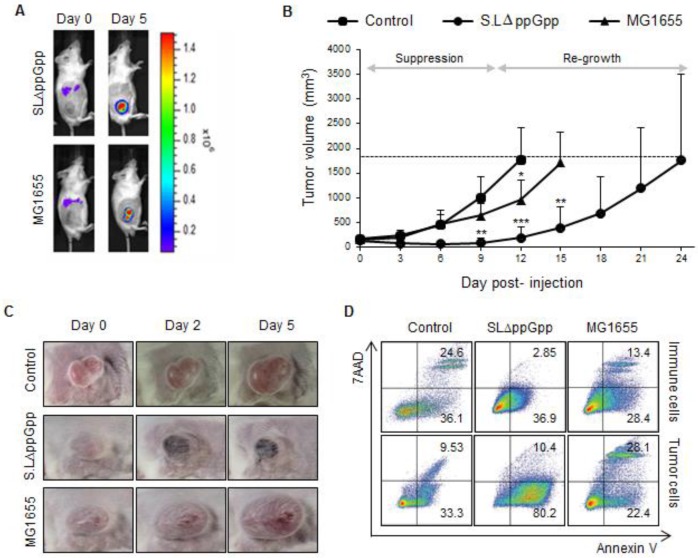
Figure 1.
Systemic injection of ΔppGpp Salmonellae (SLΔppGpp) into tumor-bearing mice induces significant growth suppression compared with E. coli (MG1655) or PBS injection. Mice (n = 5/group) were subcutaneously injected (into the right thigh) with CT26 cells (1 × 106). When the tumor volume reached 180 mm3, mice received an intravenous (i.v.) injection of PBS, MG1655 (5 × 107 CFU), or SLΔppGpp (4.5 × 107 CFU). (A) Distribution of bacteria visualized by in vivo bioluminescence imaging after injection of bacteria expressing bacterial luciferase (lux). (B) Tumor volume was then measured every 3 days until the end of experiment. The dotted line represents maximum tumor volume; mice were sacrificed when the tumor volume reached 2000mm3. Growth stages were divided into two phases based on the pattern of tumor growth after SLΔppGpp treatment; the suppression stage is the period during which the tumor stopped growing and/or shrunk in size, whereas the re-growing stage is the period during which the tumor grew again in SLΔppGpp-treated mice. (C) Images of the tumor graft in CT26-bearing mice. The shape of the tumor was noted before (0 dpi) and after treatment with PBS or bacteria (2 and 5 dpi). (D) Two days after treatment, cells were isolated from the tumor and the degree of apoptosis was measured using annexin V and 7AAD. Isolated cells were then separated into tumor cells and infiltrating immune cells to identify the cells in which apoptosis was induced. Thus, infiltrated immune cells were isolated by magnetic bead method using representative immune cell markers including CD4, CD8, CD19, B220, CD11b, CD11c, CD24, CD25, CD49b, and γδTCR. Apoptotic populations were investigated in two types of cells; immune cells and tumor cells isolated from tumor tissue. Data represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.005, and ***P < 0.001 vs. the control at Day 9 and 12 and vs. MG1655 at Day 15.