Abstract
Across invertebrates and vertebrates, steroids are potent signaling molecules that affect nearly every cell in the organism, including cells of the nervous system. Historically, researchers have focused on the genomic (or “nuclear-initiated”) effects of steroids. However, all classes of steroids also have rapid non-genomic (or “membrane-initiated”) effects, although there is far less basic knowledge of these non-genomic effects. In particular, steroids synthesized in the brain (“neurosteroids”) have genomic and non-genomic effects on behavior. Here, we review evidence that estradiol has rapid effects on aggression, an important social behavior, and on intracellular signaling cascades in relevant regions of the brain. In particular, we focus on studies of song sparrows (Melospiza melodia) and Peromyscus mice, in which estradiol has rapid behavioral effects under short photoperiods only. Furthermore, in captive Peromyscus, estrogenic compounds (THF-diols) in corncob bedding profoundly alter the rapid effects of estradiol. Environmental factors in the laboratory, such as photoperiod, diet, and bedding, are critical variables to consider in experimental design. These studies are consistent with the hypothesis that locally-produced steroids are more likely than systemic steroids to act via non-genomic mechanisms. Furthermore, these studies illustrate the dynamic balance between genomic and non-genomic signaling for estradiol, which is likely to be relevant for other steroids, behaviors, and species.
Introduction
Sex steroids are critical for the development and function of the nervous system in all vertebrate species (Micevych and Hammer 1995; Adkins-Regan 2005). Traditionally, the brain has been considered a recipient of sex steroids produced by the gonads. This idea is based on much evidence, starting with experiments on birds by Berthold (Quiring 1944) that founded the field of endocrinology (Soma 2006). In the 1970s, this idea was slightly modified when the enzyme aromatase was found in the brain (Naftolin et al. 1975). Aromatase converts testosterone (T) to estradiol (E2), and this metabolism of T represents the final stages of the action of T. The same is true of brain 5α-reductase, which converts T to 5α-dihydrotestosterone (5α-DHT) (Celotti et al. 1992). Although these developments no longer portray the brain as a passive recipient of gonadal steroids, they still assume that the brain can only metabolize T that was produced elsewhere and then reached the brain via the general circulation.
It is now clear that the adrenal glands and the brain itself play critical roles in supplying sex steroids to specific neural circuits. For example, the sex-steroid precursor dehydroepiandrosterone (DHEA) and its sulfated ester are synthesized in abundant quantities by the human adrenal cortex (Thijssen and Nieuwenhuyse 1999; Rainey et al. 2002; Beck and Handa 2004; Labrie et al. 2005). Importantly, DHEA lacks a specific intracellular steroid receptor, but DHEA can be locally metabolized into active sex steroids in peripheral tissues (e.g., prostate, breast) or regions of the brain (e.g., hypothalamus, hippocampus) with the necessary steroidogenic enzymes; this phenomenon is known as “intracrinology” (Labrie et al. 2005). Furthermore, specific regions of the brain can synthesize DHEA, T, and E2 de novo from cholesterol, independently of the gonads and adrenals (Corpechot et al. 1981; Mellon and Griffin 2002; Do Rego et al. 2009). Steroids synthesized within the nervous system, either from circulating precursors or de novo, are termed “neurosteroids” (Baulieu 1998; Corpechot et al. 1981; Schmidt et al. 2008; Do Rego et al. 2009; London et al. 2009; Schlinger and Remage-Healey 2012). These discoveries fundamentally alter our perspective, because they shift the focus from systemic levels of steroids in the blood to local levels of steroids in target tissues.
Steroid levels in the blood do not always reflect those in specific regions of the brain (Hojo et al. 2011; Taves et al. 2011b). In addition, gonadectomy does not eliminate sex steroids from some regions of the brain (Fester et al. 2011; Okamoto et al. 2012). The brain also synthesizes glucocorticoids and mineralocorticoids, and adrenalectomy does not eliminate corticosteroids from some regions (Taves et al. 2011a, 2011b). In fact, gonadectomy or adrenalectomy can lead to a compensatory increase in production of neurosteroids that maintains steroid levels in specific regions (Ye et al. 2008). Many studies have measured mRNA for steroidogenic enzymes in brain regions and neural cell lines, and it is clear that many, if not all, major classes of steroids (progestins, androgens, estrogens, glucocorticoids, mineralocorticoids) are made locally in the brain (Mellon and Griffin 2002; Tsutsui et al. 2006; Do Rego et al. 2009; Taves et al. 2011b; Fokidis et al. 2015). However, much about the behavioral functions and mechanisms of action of neurosteroids remains unknown. A growing body of evidence suggests that the mechanisms by which peripherally-produced versus centrally-produced steroids influence neurophysiology and behavior might be fundamentally different.
Genomic and non-genomic mechanisms of steroid action
The traditional view of how steroid hormones affect physiology and behavior was that steroids bind intracellular receptors that dimerize, translocate to the nucleus, bind hormone response elements on DNA, and alter the expression of genes whose protein products go on to influence behavior (Adkins-Regan 2005). This relatively slow mechanism of action for steroids was dogma for decades (Losel and Wehling 2003). However, as early as 1941, Hans Selye demonstrated that intraperitoneal injection of various steroids (e.g., deoxycorticosterone acetate, progesterone) produced deep anesthesia in rats within 15 min of administration (Seyle 1941). This groundbreaking study was the very first to show that steroids can affect physiology and behavior within minutes, and now rapid effects of all major classes of steroids are well-established (Wendler et al. 2010).
Today, the molecular mechanisms by which steroids influence cells are broadly categorized as either genomic (or “nuclear-initiated”) or non-genomic (or “membrane-initiated”) signaling (Losel and Wehling 2003; Trainor et al. 2007a; Vasudevan and Pfaff 2008; Roepke et al. 2009; Thomas 2012). Non-genomic signaling by steroids is seen across taxa, including plants, invertebrates, and vertebrates (Losel and Wehling 2003; Srivastava et al. 2005; Belkhadir and Chory 2006). While all classes of steroids have non-genomic effects on cells in the brain, the estrogens are the best characterized. The genomic mechanisms of action of E2 in the regulation of multiple forms of social behavior, especially reproductive behavior, have been thoroughly examined (McEwen 1981; Balthazart et al. 2009). For example, E2 has well-characterized effects on female reproductive behavior via changes in gene expression in hypothalamic and limbic nuclei (Christensen and Micevych 2012; Sinchak and Wagner 2012). In fact, for many years, only estrogen receptors in the diencephalon and their effects on female reproductive behavior were the focus of most research. As a result, it came as a surprise to many when it was discovered that E2 also has important effects on the brains of males, on other brain regions (e.g., hippocampus, prefrontal cortex), and on other kinds of behaviors (e.g., learning and memory, aggression) (McEwen et al. 2012). E2 is also now known to play a critical role in susceptibility to seizure (Ledoux et al. 2009), neuroinflammation (Duncan and Saldanha 2011), recovery from traumatic brain injury and stroke (Saldanha et al. 2009), and neuroprotection in Parkinson’s disease and Alzheimer’s disease (Azcoitia et al. 2011; Bourque et al. 2012; Overk et al. 2013). E2 affects nearly every part of the central nervous system and exerts genomic effects on diverse loci in the brain, from regions involved in sensory processing to cognition (Aenlle et al. 2009; Remage-Healey et al. 2012).
E2 has numerous and widespread effects on the brain and behavior within 30 min, a timeframe that is widely considered incompatible with a genomic mechanism of action. For example, E2 rapidly modulates the release of GABA, the firing of neurons, calcium flux, and the activities of kinases (such as extracellular signal-regulated kinase [ERK], phosphatidylinositol 3-kinase, and protein kinase A and C), as well as cyclic adenosine monophosphate-dependent phosphorylation (Moss and Gu 1999; Mermelstein and Micevych 2008; Micevych and Dominguez 2009; Heimovics et al. 2012). Notably, in some contexts the non-genomic effects of E2 have been shown to potentiate the genomic effects of E2 (Vasudevan and Pfaff 2008). Remarkably, some of these effects are sex-specific. For example, estradiol rapidly suppresses the release of GABA by hippocampal neurons derived from female rats but not from male rats (Huang and Woolley 2012). These non-genomic effects of E2 have become widely accepted within the past 10–15 years. However, much of this work has been done in vitro with dissociated neurons from developing animals, cultured slices of brain tissue, or with cell lines. Much less work has been done in vivo or on adult animals, with a focus on behavior.
Many of the rapid effects of experimentally administered E2 require higher doses than typically used to study the long-term effects of E2 (Cornil et al. 2006; Pradhan et al. 2008; Trainor et al. 2008). Local levels of E2 in the brain can be 50–200× higher than systemic levels of E2 in the plasma (Hojo et al. 2004; Charlier et al. 2010b, 2011; Taves et al. 2010; Overk et al. 2013). Thus, the high E2 doses necessary to elicit some of the rapid effects of E2 might produce physiologically-relevant elevations in local concentrations of E2. Also, high local E2 levels could activate putative lower-affinity estrogen receptors that are not activated by lower systemic levels of E2. Some studies have found membrane-associated estrogen receptors in the brain with relatively low affinity (Ramirez et al. 1996; Woolley 2007). Such putative membrane-associated receptors might mediate the effects of locally-produced E2. In addition, local E2 levels can rise from baseline values to very high concentrations quickly, because aromatase and other steroidogenic enzymes in the brain are rapidly regulated (Soma et al. 2004; Charlier et al. 2010a; Pradhan et al. 2010). Recently, we found that E2 levels in song sparrow’s brain tissue vary by region and season, and range from approximately 175 pg/g to approximately 400 pg/g (S. A. Heimovics, N. H. Prior, C. Q. Ma and K. K. Soma, unpublished data). Brain aromatase is rapidly regulated by calcium-dependent phosphorylation and by neurotransmitters such as dopamine and glutamate (Charlier et al. 2010a). Further, aromatase is present in presynaptic boutons (Saldanha et al. 2011) and E2 levels at these synapses might be quite high. Together, these data raise the hypothesis that neurally-synthesized E2 might act more like a neurotransmitter or neuromodulator than like a classical hormone (Balthazart et al. 2006; Remage-Healey et al. 2010; Saldanha et al. 2011).
Similarly, glucocorticoids have rapid effects on the brain and immune system (Orchinik et al. 1991; Schmidt et al. 2008, 2010). For example, glucocorticoids affect aggression via non-genomic and genomic mechanisms (Hayden-Hixson and Ferris 1991; Mikics et al. 2004; Charlier et al. 2009). Many of the rapid effects of glucocorticoids, especially on the immune system, also require high doses (Stahn and Buttgereit 2008). Locally-synthesized steroids can be produced quickly, reach high local concentrations, and rapidly bind to nearby receptors. These points suggest that locally-produced steroids are more likely than systemic steroids to act via non-genomic mechanisms (Schmidt et al. 2008).
Rapid effects of E2 on social behavior
In 1991, our understanding of the mechanisms by which E2 can influence social behavior was fundamentally altered when Hayden-Hixson and Ferris (1991) found that an infusion of E2 directed at the anterior hypothalamus could rapidly (within 20 min) increase agonistic behavior in male hamsters. Similarly, in 1999, Cross and Roselli (1999) showed that a single intraperitoneal injection of E2 could rapidly (within 35 min) increase sexual behavior (anogenital sniffing, mounting) in castrated male rats (Cross and Roselli 1999). These authors speculated that “estrogens may affect behavior in males through nontranscriptional mechanisms mediated at the membrane level” (Cross and Roselli 1999). In the years that have followed these seminal studies, it has become well-accepted that E2 can rapidly initiate intracellular signaling cascades via actions at the plasma membrane and that these intracellular signaling cascades rapidly affect behavior. Recent studies have shown that non-genomic E2 signaling is important for sexual behavior in rodents and birds (Vasudevan and Pfaff 2008; Charlier et al. 2010a; Christensen and Micevych 2012), social learning and memory in rodents (Fan et al. 2010; Choleris et al. 2012), and song perception in songbirds (Remage-Healey et al. 2010). Several excellent reviews have been published recently that highlight the rapid, non-genomic effects of E2 on these behavioral processes (Boulware et al. 2013; Cornil et al. 2013; Ervin et al. 2013; Micevych and Sinchak 2013; Remage-Healey et al. 2013; Laredo et al. 2014). Here, we focus on the evidence that E2 can exert rapid effects on aggressive behavior, and highlight research from birds and mice suggesting that environmental factors, such as photoperiod, fundamentally alter the propensity for E2 to modulate aggression via non-genomic mechanisms.
Across vertebrate species, establishment and defense of territories typically are associated with the breeding season, when plasma levels of T are elevated, and neural aromatization of circulating T is critical for the expression of aggressive behavior (Schlinger and Callard 1990; Toda et al. 2001; Matsumoto et al. 2003; Silverin et al. 2004). However, song sparrows (Melospiza melodia) of the Pacific Northwest display robust territorial aggression throughout the year (except during molt), including during the non-breeding season when the gonads are completely regressed and plasma T, 5α-DHT, androstenedione (AE), estrone (E1), and E2 are non-detectable (Wingfield and Hahn 1994; Soma et al. 1999; Soma and Wingfield 2001). This led researchers to initially hypothesize that non-breeding territorial aggression is not regulated by sex steroids in this species. This idea was supported by evidence that (1) plasma levels of T do not rise in response to a simulated territorial intrusion (STI) during the non-breeding season (Wingfield and Hahn 1994); (2) removal of the regressed testes does not reduce aggression during a STI or impair the ability to maintain a territory during the non-breeding season (Wingfield 1994); and (3) juvenile males with immature gonads successfully establish and defend territories in the non-breeding season (Wingfield and Hahn 1994; Nordby et al. 1999). However, acute and chronic treatments with aromatase inhibitors (given peripherally) significantly decrease multiple measures of non-breeding aggression (Soma et al. 1999, 2000a, 2000b). Together, these data indicate E2 is critical to the expression of aggressive behavior in both the breeding and non-breeding seasons.
Testosterone secreted by the gonads is the presumed main source of androgen substrate for brain aromatase in the breeding season. In contrast, the (indirect) source of androgen substrate for brain aromatase in the non-breeding season appears to be DHEA. In non-breeding birds, plasma levels of DHEA are several times higher than plasma levels of T and AE (Soma and Wingfield 2001; Heimovics et al. 2013). Furthermore, the seasonal patterns of plasma DHEA levels and territorial behavior are similar: both are reduced during molt (Soma and Wingfield 2001; Newman et al. 2008). The source of DHEA in the general circulation of non-breeding birds may be the regressed testes, the adrenals, or the liver (which all have high DHEA levels) (Soma and Wingfield 2001; Newman and Soma 2009, 2011) or the brain itself (the songbird brain expresses all of the enzymes required to produce DHEA de novo from cholesterol) (Tsutsui et al. 2006; Schlinger and Remage-Healey 2011). Taken together, these data suggest that the neuroendocrine control of non-breeding territoriality in song sparrows is critically dependent upon E2 made locally in the brain (i.e., neuroestrogens) (Heimovics et al. 2013; Soma et al. 2015). This is important because a growing body of evidence suggests that steroids produced locally in the brain are more likely than systemic steroids to act via rapid, non-genomic mechanisms (Schmidt et al. 2008; Cornil et al. 2013; Remage-Healey et al. 2013).
Notably, there are no major qualitative or quantitative seasonal differences in aggressive behavior during a STI in male song sparrows (Wingfield and Hahn 1994; Mukai et al. 2009; Newman and Soma 2011). When presented with a caged live decoy and playback of conspecific song, breeding and non-breeding birds sing, direct flights, approach, and spend time in close proximity to an “intruder” at similar rates. However, after a STI is terminated, breeding birds can remain aggressive for hours, while non-breeding birds stop behaving aggressively very quickly (Wingfield 1994). To date, the proximate mechanisms underlying this seasonal plasticity in the persistence of aggressive behavior (i.e., aggression during the breeding season is persistent; aggression outside of the breeding season is transient) remain unclear. As stated previously, aggressive behavior in both contexts depends upon brain aromatization, but the source of androgen substrate for aromatase appears to be seasonally variable. These data raise the hypothesis that the mechanisms by which brain-derived E2 regulates territorial aggression are also seasonally variable. Given that (1) neuroestrogens are critical to the expression of territorial aggression in the non-breeding season (2) locally-produced steroids are more likely to act non-genomically, and (3) non-genomic mechanisms are rapid and transient, one possibility is that aggression outside of the breeding season is regulated in song sparrows by E2 acting via a non-genomic mechanism.
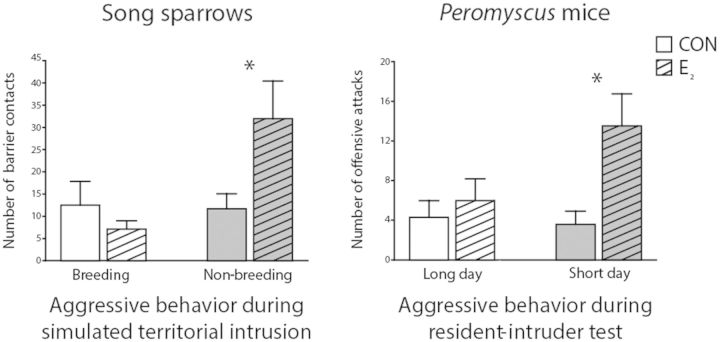
To test this hypothesis, the rapid effects of E2 on aggressive behavior were examined in male song sparrows, some in non-breeding condition and others in breeding condition, which were housed in outdoor aviaries and exposed to natural photoperiods (non-breeding ≈ 10L:14D, breeding ≈ 17L:7D) (Heimovics et al. 2015). In order to minimize the stress associated with handling, surgery, and injection, subjects were not pre-treated with an aromatase inhibitor and E2 was administered non-invasively. The E2 (300 µg cyclodextrin-encapsulated 17β-estradiol) or vehicle (2-hydroxylpropyl-β-cyclodextrin) was injected into wax-moth larvae that were subsequently fed to subjects. Ten minutes later, subjects experienced a laboratory-STI, wherein a caged live decoy was placed immediately adjacent to the subject’s home cage, and the subject and decoy were left to interact for 10 min. In the non-breeding season, E2 rapidly increased the number of times subjects contacted the wall adjacent to the decoy (“barrier contacts”) by approximately 175%; these contacts are highly aggressive in this context (Fig. 1A). Importantly, E2 had no effect on the number of times subjects contacted the opposite wall, suggesting that E2 did not affect locomotor behavior in general. In contrast, E2 had no effect on the number of barrier contacts during the breeding season. These data indicate that E2 rapidly (within 20 min) increases aggressive behavior in the non-breeding season only. Thus, the rapid behavioral effects of E2 in this paradigm are profoundly modulated by the environmental context (non-breeding season versus breeding season).
Fig. 1.
The non-genomic effects of E2 on aggressive behavior are seasonally variable in songbirds and mice. Bar graphs (mean ± SEM) representing a significant rapid (within 20–25 min) effect of E2 on (A) the number of barrier contacts made by male song sparrows during a 10-min STI and (B) the number of offensive attacks made by male Peromyscus mice during a 7-min resident-intruder test. E2 administration rapidly increased aggressive behavior in non-breeding sparrows and in mice housed under short days, but not in breeding sparrows or in mice housed under long days, suggesting that E2 promotes aggression via a non-genomic mechanism in winter. *P ≤ 0.05 (CON versus E2). Modified from Trainor et al. (2007a) and Heimovics et al. (2015).
Remarkably, the rapid effects of E2 on aggression in mice are also strongly dependent on an environmental cue, photoperiod. Administration of E2 increases male aggressive behavior by approximately 200% within 25 min in two species of Peromyscus mice housed under a short (8L:16D, winter-like) photoperiod (Trainor et al. 2007a, 2008) (Fig. 1B). This same treatment has no effect in mice housed under a long (16L:8D, spring-like) photoperiod. These data suggest that the non-genomic effects of E2 on behavior may predominate on short days. Interestingly, the effect of photoperiod on E2 action in Peromyscus is independent of changes in gonadal hormones. Old-field mice (P. polionotus) housed under short days have reduced testicular size and lower levels of circulating T (Trainor et al. 2007b). However, California mice (P. californicus) housed under short days have similar testicular size and levels of circulating T as those housed under long days. In both species, injections of E2 rapidly increase aggressive behavior only under short days.
In contrast, microarray and real-time PCR show that genes regulated by estrogen response elements (EREs) (identified as regulated by EREs via ChIP promoter microarray analysis) are up-regulated in the bed nucleus of the stria terminalis (a brain region that regulates aggression) of mice housed under long days when compared with mice housed under short days (Trainor et al. 2007a). Further, the effect of photostimulation on XRCC1 (an estrogen-dependent transcript) can be eliminated by an aromatase inhibitor (Trainor et al. 2007a). While it is still not clear whether this gene affects behavior, these data suggest that the genomic effects of E2 may predominate on long days. As stated previously, there is some evidence to show that non-genomic and genomic mechanisms of E2 interact (Vasudevan and Pfaff 2008) but these data strongly suggest that photoperiod shifts the dynamic balance between genomic and non-genomic E2 signaling.
Importantly, E2 regulation of aggression in Peromyscus is also highly sensitive to the presence of estrogen-like compounds in the environment. The Peromyscus studies described above were conducted with mice housed in cages lined with corncob bedding. Corncob contains tetrahydrofuran diols (THF-diols), which have estrogen-like properties (Markaverich et al. 2002a, 2002b; Mani et al. 2005; Sakhai et al. 2013). The corncob bedding is ingested by rodents, increasing THF-diols in the blood and reducing ERα immunoreactivity in the hypothalamus and phosphorylated ERK throughout the brain (Villalon Landeros et al. 2012). Surprisingly, when short-day Peromyscus were housed instead on a cardboard-based bedding, E2 treatment rapidly reduced aggression rather than increasing it. However, consistent with the previous studies using corncob bedding, E2 rapidly modulated aggression during short days but not long days, and this rapid effect of E2 was not blocked by the protein synthesis inhibitor cycloheximide (Laredo et al. 2013). Protein synthesis is considered to be an important component of the genomic actions of steroid hormones (O'Malley and Means 1974). The striking effects of type of bedding on E2 action have the potential to impact a wide range of research programs utilizing rodents in a laboratory setting. Many animal facilities are switching to corncob bedding, sometimes without input from researchers (Sakhai et al. 2013). Although the bedding in cages often is not considered in experimental design, these examples highlight the sensitivity of estrogen signaling to environmental factors such as photoperiod, bedding, diet, or endocrine disruptors in plastic cages or water bottles (Laredo et al. 2014).
Notably, data from another songbird (Gambel’s white-crowned sparrow, Zonotrichia leucophrys pugetensis) show that an environmental factor can alter the rapid (non-genomic) effects of corticosterone (CORT) on behavior. Specifically, Breuner and Wingfield (2000) found that non-invasive administration of CORT rapidly increased perch-hopping (activity) in long-day (20L:4D) but not in short-day (8L:16D) subjects. Thus, it appears that photoperiod can profoundly influence the mechanisms by which both E2 and CORT influence the brain and behavior. The effect of photoperiod on rapid, non-genomic effects of other steroid hormones on behavior remains to be explored. Importantly, while seasonality and photoperiodism are pervasive in nature, they are largely neglected in behavioral neuroscience research in the laboratory. Much of our understanding of how the brain regulates behavior comes from traditional rodent models housed on an invariant and intermediate photoperiod (12L:12D). Thus, potential effects of photoperiod on neuroendocrine mechanisms may be greatly underappreciated (Nelson and Trainor 2007; Soma et al. 2008; Newman et al. 2013).
Mechanisms underlying the shift to non-genomic signaling
The proximate mechanisms that underlie seasonal differences in the rapid, non-genomic effects of E2 on aggressive behavior remain unclear. One possibility is that photoperiod drives seasonal changes in gonadal secretion of T, which then affects E2 signaling in the brain. However, it is worth noting that photoperiod does not alter circulating levels of T in California mice, yet the non-genomic effects of E2 on aggressive behavior are nonetheless only seen in short-day subjects (Trainor et al. 2008; Laredo et al. 2013). Thus, photoperiod might alter E2 signaling in the brain via other pathways.
It is widely known that photoperiod affects the nocturnal synthesis and secretion of melatonin by the pineal gland (Reiter 1980; Bartness and Goldman 1988). Photoperiod also affects the diurnal synthesis and secretion of vitamin D by the skin (Norman 1998; Parker et al. 2002). Thus, it is possible that the effects of photoperiod on E2 signaling are mediated by melatonin and/or vitamin D. For example, melatonin and/or vitamin D could potentially influence the trafficking of classical estrogen receptors to the plasma membrane, expression of non-classical estrogen receptors, or the ability of these receptors to activate intracellular signaling cascades (see below).
Classical estrogen receptors
It appears unlikely that photoperiodic changes in E2 signaling are mediated by simple alterations in the expression of ERα or ERβ. Three species of rodents with elevated aggression under short days (Siberian hamsters, old-field mice, and deer mice) all have increased ERα immunoreactivity in the BNST under short days (Trainor et al. 2007b; Kramer et al. 2008). However, California mice also have elevated aggression under short days, yet ERα immunoreactivity in the BNST is not increased (Trainor et al. 2008). Estrogens act rapidly to modulate aggression under short days in both old-field mice and California mice, so increased neural ERα immunoreactivity does not appear to be a pre-requisite.
One question that has not yet been examined is whether photoperiod modulates the trafficking of classical estrogen receptors. Levels of ERα and ERβ associated with the plasma membrane are dynamic; these receptors are trafficked to, and then sequestered from, the plasma membrane as needed (Milner et al. 2001, 2005; Mermelstein and Micevych 2008; Dominguez and Micevych 2010; Mitterling et al. 2010; Hammes and Levin 2011). Based on the results of behavioral studies, we would expect higher levels of classical estrogen receptors at the plasma membrane under short photoperiods. Future studies should examine this possibility.
Non-classical estrogen receptors
Currently, there is some evidence that behavior is regulated by a membrane-bound estrogen receptor: G-protein-coupled estrogen receptor-1 (GPER-1, previously known as GPR30). GPER-1 was first identified in breast cancer cells and then later identified in mammalian cortex, hippocampus, and hypothalamus (Thomas et al. 2005; Prossnitz and Barton 2011; Filardo and Thomas 2012). So far, no study has directly tested whether GPER-1 modulates aggressive behavior.
However, recent studies show that GPER-1 is an important modulator of behavior (Kastenberger et al. 2012b; Anchan et al. 2014a, 2014b; Hart et al. 2014). In intact male C57Bl/6N mice, administration of a GPER-1 agonist (G-1, 1 mg/kg) has anxiogenic effects in the open field test, elevated plus maze, and light-dark box test (Kastenberger et al. 2012a, 2012b). Increased anxiety-like behavior has been linked to elevated levels of aggression in mice (Kudryavtseva et al. 2014), suggesting that GPER-1 could modulate aggression as well. However, in a separate study, very different results were observed in castrated male mice. In castrated mice, administration of G-1 (∼125 µg/kg) has anxiolytic effects (Hart et al. 2014). These results suggest that the effects of GPER-1 activation may depend on gonadal hormones, which can upregulate or downregulate steroid receptors in a region-specific manner (Brown et al. 1996). It is still unclear how GPER-1 expression is modulated by gonadal hormones. Alternatively, the effects of G-1 on anxiety might vary with dose; the dose of G-1 that induced anxiogenic effects was almost 10 times higher than the dose that induced anxiolytic effects. Further systematic research will be needed to determine what role, if any, GPER-1 plays in modulating aggressive behavior.
Intracellular signaling cascades
In order for membrane-associated estrogen receptors to rapidly modulate neural activity, a second messenger system must be activated. Several candidate pathways have been investigated. Activation of ERK has been considered a good candidate, because phosphorylated ERK (pERK) can alter neuronal excitability and the release of neurotransmitter (Selcher et al. 2003). Estradiol can rapidly induce phosphorylation of ERK in the brain, an effect that is blunted in female ERα and ERβ knockout mice (Abraham et al. 2004). Injection of E2-BSA also induces phosphorylation of ERK in spinal cord tissue from female rats (Nag and Mokha 2014), suggesting that membrane-associated receptors mediate the effects of E2 on the activation of ERK.
In Peromyscus, E2 rapidly regulates aggression under short days. Interestingly, engaging in aggression tests increases the number of pERK-positive cells in the BNST and MEA of male California mice, but only under short days (Trainor et al. 2010). Furthermore, the number of pERK-positive cells in these regions was positively correlated with aggression. The mice in these studies were intact and were not treated with hormones. Hormone manipulation studies suggest that the relationships between E2, ERK activity, and aggression are complex. In one study, male California mice were housed under short days (and on cardboard-based bedding) and treated with an aromatase inhibitor (fadrozole) for 10 days to reduce endogenous estrogens. Notably, treatment with fadrozole increased aggressive behavior and also decreased the number of pERK-positive cells in the BNST and MEA (Villalon Landeros et al. 2012). This result indicates that increased aggressive behavior can also be associated with decreased levels of ERK activation in the BNST and MEA of male California mice.
The rapid effects of E2 on intracellular signaling have also been examined in song sparrows. Subjects that were in breeding or non-breeding condition were treated with an aromatase inhibitor (fadrozole) for 10 days, then injected with saline or E2 (500 μg/kg s.c.), and 15 min later their brains were harvested. Injections of E2 decreased pERK immunoreactivity in nucleus taeniae (TnA, part of the extended amygdala) and had no effect on pERK in the BNST (Heimovics et al. 2012). Notably, this rapid inhibitory effect of E2 on pERK in TnA was observed both in breeding and in non-breeding birds, whereas a rapid stimulatory effect of E2 on aggressive behavior was only seen in non-breeding birds. Thus, studies of rodents and birds have shown that the effects of E2 on pERK in the BNST and amygdala can be dissociated from the effects of E2 on aggression. While the ERK activity in the extended amygdala is sensitive to estrogen signaling, it might not be a critical mediator of the rapid effects of estrogens on aggression.
An important consideration is that experiments examining the effects of E2 on ERK were conducted on animals treated with aromatase inhibitors. Although this approach provides control over levels of estrogens, sustained aromatase inhibition could result in important changes in ERK activity. For example, the effects of the activation of ERK on cellular function are very different depending on the time-course of activation. Transient activation of ERK can modulate neuronal activity and the release of neurotransmitters (Selcher et al. 2003). In contrast, sustained activation of ERK induces long-term changes in gene expression and cellular function (Marshall 1995; Grewal et al. 1999). It is possible that long-term aromatase inhibition may have different effects on ERK activity versus short-term aromatase inhibition. Further, the putative discrepancy between pERK downregulation and the induction of aggression in the song sparrow could be due to the concentrations of neuroestrogens when aromatase inhibitors are administered. For example, too little or too much neuroestrogen can inhibit sociosexual behavior in the quail (Ubuka and Tsutsui 2014). Thus, it would be useful to compare the effects of long-term inhibition of aromatase versus short-term inhibition both on behavior and on ERK activity.
As stated above, E2 can rapidly modulate phosphorylation of ERK, which phosphorylates a variety of proteins, including cAMP response element binding protein (CREB), a transcription factor that binds to cAMP response elements (CREs) in gene promoter regions. Thus, membrane-initiated E2 signaling can affect the expression of CRE-containing genes (e.g., c-fos, egr-1). Interestingly, E2 rapidly decreases pCREB in the medial preoptic nucleus (POM) in non-breeding (but not in breeding) song sparrows (Heimovics et al. 2012). Notably, this pattern mirrors the rapid effect of E2 on aggressive behavior in non-breeding (but not in breeding) song sparrows (Heimovics et al. 2015). The POM is best known for its role in regulating the sexual behavior of males (Balthazart and Ball 2007), but is also strongly implicated in the regulation of aggressive behavior in birds (Riters 2011; Pan et al. 2010; Patil and Brid 2010).
Finally, E2 can rapidly modulate the activity of enzymes involved in neurotransmitter synthesis, in part via activation of ERK. Tyrosine hydroxylase (TH) is a rate-limiting enzyme that is necessary for the synthesis of catecholamines, including dopamine and norepinephrine. Phosphorylation at four different serines in TH appears to facilitate the activity of TH in the hypothalamus (Liu and Arbogast 2008). Several studies have demonstrated that E2 rapidly modulates phosphorylation of TH, primarily in rodent striatum. Ovariectomized rats injected with E2 had increased phosphorylation of TH and increased dopamine synthesis (Pasqualini et al. 1995) and increased release of dopamine in striatum within 15–20 min (Becker 1990). Interestingly, E2 injections do not increase striatal dopamine release in castrated rats (Castner et al. 1993). In these studies, gonadal status has an important effect on how an acute injection of E2 impacts dopamine release. In intact female rats, the release of striatal dopamine fluctuates across the estrous cycle, peaking at estrus and proestrus (Xiao and Becker 1994). Ovariectomy reduces the release of dopamine to levels equivalent to diestrus. In contrast, castration had no effect on the release of dopamine in males. Most studies examining rapid effects of E2 on dopaminergic function have been conducted with gonadectomized animals, which provides more experimental control over levels of E2. However, gonadectomy might alter how the brain responds to a rapid pulse of E2. In addition, E2 may have different effects on TH in the striatum versus in the hypothalamus. One study examined the effect of E2 on dopamine synthesis in slices of the hypothalamus collected from ovariectomized rats (Pasqualini et al. 1993). Treatment with E2 rapidly decreased phosphorylation of TH and reduced dopamine synthesis. Consistent with this observation, E2 injected into intact male song sparrows reduced the number of pTH-positive cells in the ventromedial hypothalamus but not in the lateral septum (Heimovics et al. 2012). These data suggest that the downstream effects of E2 on dopaminergic neurotransmission are region-specific. Given the well-documented role of dopamine in regulating highly motivated, goal-directed behaviors, the interaction between non-genomic E2 signaling and dopaminergic neurotransmission in the neural control of aggression (a highly goal-directed behavior) will be an important avenue for future research.
Conclusions
Many classes of steroids are produced locally and act locally within a variety of tissues, including the brain (Taves et al. 2011a, 2015; Fokidis et al. 2015). A growing body of evidence from across taxa suggests that these neurosteroids act via rapid and transient non-genomic mechanisms (Woolley 2007; Schmidt et al. 2008; Cornil et al. 2013; Remage-Healey et al. 2013). Data from birds and mice indicate that the non-genomic effects of E2 on aggressive behavior predominate during the non-breeding season. In the non-breeding season, food is less abundant and low ambient temperatures impose large metabolic costs (Rogers et al. 1991; Rogers 1995; Heimovics et al. 2013). The additional metabolic costs of engaging in prolonged aggressive behavior can be substantial (Haller 1995; Muehlenbein and Watts 2010). Thus, a shift to non-genomic steroid regulation of aggressive behavior in the non-breeding season may have evolved because there is adaptive value in transient expression of aggressive behavior at that time (Heimovics et al. 2015).
Natural and synthetic steroids are among the most widely prescribed medications in the world (e.g., birth control, hormone replacement therapy). In addition, many drugs are used to reduce steroid synthesis or signaling (e.g., to treat breast cancer). Despite this widespread usage, much is unknown about how steroids act on the brain and other organs. This issue is relevant for designing better steroid treatments for estrogen-sensitive brain diseases, such as Alzheimer’s disease (Pike et al. 2009; Barron and Pike 2012; Overk et al. 2013). Treatments targeting the non-genomic effects of neurosteroids may be particularly useful in patients with an endocrine state characterized by low levels of sex steroids in the blood (e.g., most of the elderly; men undergoing androgen deprivation therapy for prostate cancer). Studies of neuroestrogens and non-genomic E2 signaling will also inform the development of drugs that modulate membrane estrogen receptors, regulate signaling pathways used by E2, or stimulate E2 synthesis in specific regions of the brain. Such developments would greatly reduce the negative side effects of systemic steroid treatments on peripheral organs, which is currently a major concern.
Funding
This work was supported by the Canadian Institute for Health Research (CIHR) and Michael Smith Foundation for Health Research postdoctoral fellowships [to S.A.H.], National Institutes of Health Grant [MH85069 to B.C.T.], and CIHR Operating Grant [133606 to K.K.S].
Acknowledgments
The authors would like to thank Drs Rebecca Calisi and Colin Saldanha for organizing the “Neurohormones, Brain and Behavior: A Comparative Approach to Rapid Neuroendocrine Function” symposium at the 2015 SICB annual meeting.
References
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Hormones and animal social behavior. Princeton: Princeton University Press; 2005. [Google Scholar]
- Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–45. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchan D, Clark S, Pollard K, Vasudevan N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav. 2014a;4:51–9. doi: 10.1002/brb3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anchan D, Gafur A, Sano K, Ogawa S, Vasudevan N. Activation of the GPR30 receptor promotes lordosis in female mice. Neuroendocrinology. 2014b;100:71–80. doi: 10.1159/000365574. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22:467–73. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–78. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF. Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior in quail. J Exp Zool A Ecol Genet Physiol. 2009;311:323–45. doi: 10.1002/jez.464. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–97. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. Am J Physiol. 1988;255:R812–22. doi: 10.1152/ajpregu.1988.255.5.R812. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Beck SG, Handa RJ. Dehydroepiandrosterone (DHEA): a misunderstood adrenal hormone and spine-tingling neurosteroid? Endocrinology. 2004;145:1039–41. doi: 10.1210/en.2003-1703. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–94. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front Neuroendocrinol. 2012;33:169–78. doi: 10.1016/j.yfrne.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC. Rapid behavioral response to corticosterone varies with photoperiod and dose. Horm Behav. 2000;37:23–30. doi: 10.1006/hbeh.1999.1554. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Martini L. 5α-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol. 1992;13:163–215. [PubMed] [Google Scholar]
- Charlier TD, Cornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim Biophys Acta. 2010a;1800:1094–105. doi: 10.1016/j.bbagen.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–53. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010b;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Underhill C, Hammond GL, Soma KK. Effects of aggressive encounters on plasma corticosteroid-binding globulin and its ligands in white-crowned sparrows. Horm Behav. 2009;56:339–47. doi: 10.1016/j.yhbeh.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. 2012;33:140–59. doi: 10.1016/j.yfrne.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153:3872–7. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–23. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Seredynski AL, de Bournonville C, Dickens MJ, Charlier TD, Ball GF, Balthazart J. Rapid control of reproductive behaviour by locally synthesised oestrogens: focus on aromatase. J Neuroendocrinol. 2013;25:1070–8. doi: 10.1111/jne.12062. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78:4704–7. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol. 1999;276:R1346–50. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–96. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KA, Saldanha CJ. Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J Neuroinflammation. 2011;8:81. doi: 10.1186/1742-2094-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol. 2013;25:1116–32. doi: 10.1111/jne.12079. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285–94. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–62. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokidis HB, Adomat HH, Kharmate G, Hosseini-Beheshti E, Guns ES, Soma KK. Regulation of local steroidogenesis in the brain and in prostate cancer: lessons learned from interdisciplinary collaboration. Front Neuroendocrinol. 2015;36C:108–29. doi: 10.1016/j.yfrne.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–53. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Haller J. Biochemical background for an analysis of cost-benefit interrelations in aggression. Neurosci Biobehav Rev. 1995;19:599–604. doi: 10.1016/0149-7634(95)00053-4. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–95. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, Clark SM, Vasudevan N. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids. 2014;81:49–56. doi: 10.1016/j.steroids.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiol Behav. 1991;50:793–9. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Ferris JK, Soma KK. Non-invasive administration of 17beta-estradiol rapidly increases aggressive behavior in non-breeding, but not breeding, male song sparrows. Horm Behav. 2015;69:31–8. doi: 10.1016/j.yhbeh.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Fokidis HB, Soma KK. Brain aromatase and territorial aggression across the seasons in male song sparrows. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens, and behavior. Oxford; New York: Oxford University Press; 2013. pp. 199–220. [Google Scholar]
- Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153:1364–76. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, et al. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenberger I, Lutsch C, Herzog H, Schwarzer C. Influence of sex and genetic background on anxiety-related and stress-induced behaviour of prodynorphin-deficient mice. PLoS One. 2012a;7:e34251. doi: 10.1371/journal.pone.0034251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenberger I, Lutsch C, Schwarzer C. Activation of the G-protein-coupled receptor GPR30 induces anxiogenic effects in mice, similar to oestradiol. Psychopharmacology (Berl) 2012b;221:527–35. doi: 10.1007/s00213-011-2599-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Simmons JL, Freeman DA. Photoperiod alters central distribution of estrogen receptor alpha in brain regions that regulate aggression. Horm Behav. 2008;53:358–65. doi: 10.1016/j.yhbeh.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Smagin DA, Kovalenko IL, Vishnivetskaya GB. Repeated positive fighting experience in male inbred mice. Nat Protocols. 2014;9:2705–17. doi: 10.1038/nprot.2014.156. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–96. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Laredo SA, Villalon Landeros R, Dooley JC, Steinman MQ, Orr V, Silva AL, Crean KK, Robles CF, Trainor BC. Nongenomic effects of estradiol on aggression under short day photoperiods. Horm Behav. 2013;64:557–65. doi: 10.1016/j.yhbeh.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo SA, Villalon Landeros R, Trainor BC. Rapid effects of estrogens on behavior: environmental modulation and molecular mechanisms. Front Neuroendocrinol. 2014;35:447–58. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29:1457–68. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Arbogast LA. Phosphorylation state of tyrosine hydroxylase in the stalk-median eminence is decreased by progesterone in cycling female rats. Endocrinology. 2008;149:1462–9. doi: 10.1210/en.2007-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–14. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols) Steroids. 2005;70:750–4. doi: 10.1016/j.steroids.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O'Malley B, Faith R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect. 2002a;110:169–77. doi: 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaverich BM, Alejandro MA, Markaverich D, Zitzow L, Casajuna N, Camarao N, Hill J, Bhirdo K, Faith R, Turk J, et al. Identification of an endocrine disrupting agent from corn with mitogenic activity. Biochem Biophys Res Commun. 2002b;291:692–700. doi: 10.1006/bbrc.2002.6499. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–24. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neural gonadal steroid actions. Science. 1981;211:1303–11. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Synthesis, regulation, and function of neurosteroids. Endocr Res. 2002;28:463. doi: 10.1081/erc-120016823. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–24. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–27. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Temporal and concentration-dependent effects of oestradiol on neural pathways mediating sexual receptivity. J Neuroendocrinol. 2013;25:1012–23. doi: 10.1111/jne.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Hammer R. Neurobiological effects of sex steroid hormones. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Mikics E, Kruk MR, Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology. 2004;29:618–35. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–43. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, Gu Q. Estrogen: mechanisms for a rapid action in CA1 hippocampal neurons. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Watts DP. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc Med. 2010;4:21. doi: 10.1186/1751-0759-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Replogle K, Drnevich J, Wang G, Wacker D, Band M, Clayton DF, Wingfield JC. Seasonal differences of gene expression profiles in song sparrow (Melospiza melodia) hypothalamus in relation to territorial aggression. PLoS One. 2009;4:e8182. doi: 10.1371/journal.pone.0008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan K, Davies I, Reddy V, Flores F, Petro Z, Kuhn M, White R, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:255–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nag S, Mokha SS. Activation of a Gq-coupled membrane estrogen receptor rapidly attenuates alpha2-adrenoceptor-induced antinociception via an ERK I/II-dependent, non-genomic mechanism in the female rat. Neuroscience. 2014;267:122–34. doi: 10.1016/j.neuroscience.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Newman AE, Pradhan DS, Soma KK. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology. 2008;149:2537–45. doi: 10.1210/en.2007-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AE, Soma KK. Corticosterone and dehydroepiandrosterone in songbird plasma and brain: effects of season and acute stress. Eur J Neurosci. 2009;29:1905–14. doi: 10.1111/j.1460-9568.2009.06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AE, Soma KK. Aggressive interactions differentially modulate local and systemic levels of corticosterone and DHEA in a wild songbird. Horm Behav. 2011;60:389–96. doi: 10.1016/j.yhbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Newman AE, Zanette LY, Clinchy M, Goodenough N, Soma KK. Stress in the wild: chronic predator pressure and acute restraint affect plasma DHEA and corticosterone levels in a songbird. Stress. 2013;16:363–7. doi: 10.3109/10253890.2012.723076. [DOI] [PubMed] [Google Scholar]
- Nordby JC, Campbell SE, Beecher MD. Ecological correlates of song learning in song sparrows. Behav Ecol. 1999;10:287–97. [Google Scholar]
- Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108–10. doi: 10.1093/ajcn/67.6.1108. [DOI] [PubMed] [Google Scholar]
- O'Malley BW, Means AR. Female steroid hormones and target cell nuclei. Science. 1974;183:610–20. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Hojo Y, Inoue K, Matsui T, Kawato S, McEwen BS, Soya H. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA. 2012;109:13100–5. doi: 10.1073/pnas.1210023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–51. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Overk CR, Perez SE, Ma C, Taves MD, Soma KK, Mufson EJ. Sex steroid levels and AD-like pathology in 3xTgAD mice. J Neuroendocrinol. 2013;25:131–44. doi: 10.1111/j.1365-2826.2012.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Xu L, Young KA, Wang Z, Zhang Z. Agonistic encounters and brain activation in dominant and subordinate male greater long-tailed hamsters. Horm Behav. 2010;58:478–84. doi: 10.1016/j.yhbeh.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JE, Timm KI, Smith BB, Van Saun RJ, Winters KM, Sukon P, Snow CM. Seasonal interaction of serum vitamin D concentration and bone density in alpacas. Am J Vet Res. 2002;63:948–53. doi: 10.2460/ajvr.2002.63.948. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Guibert B, Leviel V. Short-term inhibitory effect of estradiol on tyrosine hydroxylase activity in tuberoinfundibular dopaminergic neurons in vitro. J Neurochem. 1993;60:1707–13. doi: 10.1111/j.1471-4159.1993.tb13394.x. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65:1651–7. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- Patil SN, Brid SV. Relative role of neural substrates in the aggressive behavior of rats. J Basic Clin Physiol Pharmacol. 2010;21:357–67. doi: 10.1515/jbcpp.2010.21.4.357. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–58. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–9. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104:244–53. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–26. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiring DP. Transplantation of testes (by A.A. Berthold) Bull Hist Med. 1944;16:399–401. [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–9. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–98. doi: 10.1007/BF02088175. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–31. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA. 2010;107:3852–7. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–31. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol. 2013;25:1024–31. doi: 10.1111/jne.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neurosci Biobehav Rev. 2011;35:1837–45. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–70. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CM. Experimental-evidence for temperature-dependent winter lipid storage in the dark-eyed Junco (Junco hyemalis oreganus) and song sparrow (Melospiza melodia morphna) Physiol Zool. 1995;68:277–89. [Google Scholar]
- Rogers CM, Smith JNM, Hochachka WM, Cassidy A, Taitt MJ, Arcese P, Schluter D. Spatial variation in winter survival of song sparrows Melospiza melodia. Ornis Scandinavica. 1991;22:387–95. [Google Scholar]
- Sakhai SA, Preslik J, Francis DD. Influence of housing variables on the development of stress-sensitive behaviors in the rat. Physiol Behav. 2013;120:156–63. doi: 10.1016/j.physbeh.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–18. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–49. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen Comp Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Remage-Healey L. Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol. 2011;24:16–21. doi: 10.1111/j.1365-2826.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Remage-Healey L. Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol. 2012;24:16–21. doi: 10.1111/j.1365-2826.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Malisch JL, Breuner CW, Soma KK. Corticosterone and cortisol binding sites in plasma, immune organs and brain of developing zebra finches: intracellular and membrane-associated receptors. Brain Behav Immun. 2010;24:908–18. doi: 10.1016/j.bbi.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol. 2008;157:266–74. doi: 10.1016/j.ygcen.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyle H. Anesthetic effect of steroid hormones. Exp Biol Med. 1941;46:116–21. [Google Scholar]
- Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–34. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–63. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–51. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–77. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: insights from comparative studies of aggression. J Steroid Biochem Mol Biol. 2015;145C:261–72. doi: 10.1016/j.jsbmb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–89. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115:442–53. doi: 10.1006/gcen.1999.7334. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. J Comp Physiol A. 2000a;186:759–69. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc Biol Sci. 2000b;267:1089–96. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–55. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–55. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011a;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Ma C, Heimovics SA, Saldanha CJ, Soma KK. Measurement of steroid concentrations in brain tissue: methodological considerations. Front Endocrinol (Lausanne) 2011b;2:39. doi: 10.3389/fendo.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology. 2015;156:511–22. doi: 10.1210/en.2013-1606. [DOI] [PubMed] [Google Scholar]
- Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK. Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PLoS One. 2010;5:e15727. doi: 10.1371/journal.pone.0015727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen JHH, Nieuwenhuyse H. DHEA: a comprehensive review. New York: Parthenon Publishing Group; 1999. [Google Scholar]
- Thomas P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen Comp Endocrinol. 2012;175:367–83. doi: 10.1016/j.ygcen.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–20. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WH, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–36. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–9. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci USA. 2007a;104:9840–5. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Rowland MR, Nelson RJ. Photoperiod affects estrogen receptor alpha, estrogen receptor beta and aggressive behavior. Eur J Neurosci. 2007b;26:207–18. doi: 10.1111/j.1460-9568.2007.05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Matsunaga M, Miyabara H, Ukena K. Neurosteroid biosynthesis in the quail brain: a review. J Exp Zool A Comp Exp Biol. 2006;305:733–42. doi: 10.1002/jez.a.302. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Tsutsui K. Gonadotropin-inhibitory hormone inhibits aggressive behavior of male quail by increasing neuroestrogen synthesis in the brain beyond its optimum concentration. Gen Comp Endocrinol. 2014 doi: 10.1016/j.ygcen.2014.03.014. 79–39–53. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–57. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-alpha expression in the brain. Endocrinology. 2012;153:949–53. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler A, Baldi E, Harvey BJ, Nadal A, Norman A, Wehling M. Position paper: rapid responses to steroids: current status and future prospects. Eur J Endocrinol. 2010;162:825–30. doi: 10.1530/EJE-09-1072. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav. 1994;28:1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hahn TP. Testosterone and territorial behavior in sedentary and migratory sparrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Ye P, Kenyon CJ, Mackenzie SM, Nichol K, Seckl JR, Fraser R, Connell JM, Davies E. Effects of ACTH, dexamethasone, and adrenalectomy on 11beta-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol. 2008;196:305–11. doi: 10.1677/JOE-07-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]