Figure 1.
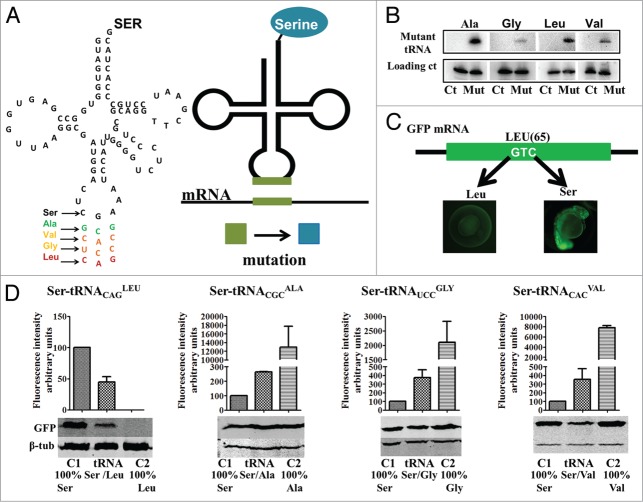
Proteome mutagenesis using misreading tRNAs (A) A Ser-tRNA gene was mutated in the anticodon region by site directed mutagenesis to produced tRNAs that insert Ser at alanine (GCG), valine (GTG), glycine (GGA) and leucine (CTG) codons. Our method takes advantage of the fact that the seryl-tRNA-synthetase (SerRS) does not rely on the anticodon sequence to recognize the Ser tRNAs, thus allowing the mutant tRNA to be charged with Ser. These mutant tRNAs incorporate Ser at non-cognate Ala, Gly, Val and Leu sites producing mutant proteins. (B) Northern blot analysis showing that all mutant tRNAs are expressed and processed. (C) A GFP reporter was used to quantify the level of Ser misincorporation at non-cognate sites. The residue Ser 65 is critical for GFP fluorescence, insertion of another amino acid at residue 65 shifts overall fluorescence. (D) The mutant tRNAs were able to partially restore wild type GFP fluorescence levels. Western blot analysis of GFP expression was used to normalize fluorescence levels; it also shows that Ser insertion at position 65 is able the restore GFP synthesis or stability in the case of the Leu mutant. Data are presented as the mean ± SD (n = 4). Fluorescence units are relative to Control 1. Control 1 refers to 100% insertion of serine at position 65. Control 2 refers to insertion of 100% insertion of the mutated amino acids tested.