Abstract
The objective of the study was to define and quantitatively evaluate the fascicular ratio (FR) on magnetic resonance imaging (MRI) in patients with peripheral neuropathies compared with healthy controls.
Forty control subjects (20 women, 20 men; age, 44.6 ± 13.4 years) and 40 patients with peripheral neuropathy (22 women, 18 men; age, 50.3 ± 10.2 years) were examined with a standard 3T MRI protocol. With customized software (with semiautomatic and automatic interface), the hypointense and hyperintense areas of the peripheral nerves corresponding to fascicular and nonfascicular tissue were examined on T1-weighted sequences. The ratio of fascicular pixels to total pixels was called FR. Correlation with FR calculated on high-resolution ultrasound was performed. The statistical analysis included the Mann–Whitney U test of controls versus patients, the receiver operating characteristic (ROC) analysis, and the subgroup analysis of patients according to etiologies of neuropathy. Intraobserver and interobserver agreement was calculated based on the evaluation made by 3 readers. Finally, a complete automatic evaluation was performed.
On MRI, FRs were significantly increased in patients compared with controls (FR, 76.7 ± 15.1 vs 56 ± 12.3; P < 0.0001 for the semiautomatic interface; and FR 66.3 ± 17.5 vs 47.8 ± 18.4; P < 0.0001 for the automatic interface). The increase in FR was caused mainly by an increase in the hypointense part of the nerve. This observation was valid for all causes of neuropathies. ROC analysis found an area under the curve of 0.75 (95% confidence interval, 0.44–0.81) for FR to discriminate neuropathy from control. The correlation coefficient between MRI and ultrasound was significant (r = 0.49; 95% confidence interval for r, 0.21–0.70; P = 0.012).
With the semiautomated evaluation, the mean intraobserver agreement was good (K = 0.86). The interobserver agreements were also good (reader 1 vs reader 2, k = 0.71; reader 2 vs reader 3, k = 0.78; reader 3 vs reader 1, k = 0.71). There were no statistically significant differences between the results obtained using the 2 methods.
FR calculation on MRI is feasible, and it may be used in adjunct to standard MRI evaluation in peripheral nerve disorders.
INTRODUCTION
The peripheral nervous system is increasingly being investigated using magnetic resonance imaging (MRI).1–4 Electrophysiology is still the clinical “gold standard” for nerve assessment, but rapidly accumulating literature exists comparing this modality with both ultrasound (US) and MRI,5–8 which are being used more and more frequently in various clinical settings to evaluate the peripheral nervous system (eg, for inherited disorders, entrapment syndromes, traumas, and tumors), thereby influencing the diagnosis and finally the clinical care of the patient.5–8
Structural analysis of the peripheral nerve includes evaluation of the nerve’s inner texture based on a subjective evaluation. In several neuropathies, changes in the inner texture, which are consistent with the loss of the fascicular pattern due to intraneural edema, fibrosis, or fascicular alterations, have been considered signs of pathology both on US and MRI.9–12
On US, the evaluation of the inner texture of the nerve has been named “nerve density.”10 In this article, we will use the term fascicular ratio (FR) for both US and MRI instead of “nerve density.” The use of nerve echogenicity as an indicator of nerve pathology is based on the concept that nervous tissue can be broadly divided into 2 different categories of echogenicity. Fascicular tissue, which mainly consists of nerve fibers, poorly reflects the US beam because of its high water content and less number of interfaces, thereby appearing hypoechogenic. Nonfascicular tissue, which consists of connective tissue, blood, and lymphatic vessels, reflects the US beam and is hyperechogenic.10 Similarly, we believe that the relationship between fascicular and nonfascicular tissue may also be calculated on MRI. On MRI, the fascicular tissue is relatively lipid-poor and appears generally hypointense on T1-weighted sequences or slightly hyperintense on T2-weighted sequences.13,14 In contrast, the nonfascicular tissue appears hypointense on T2-weighted sequences with fat saturation or hyperintense on T1-weighted sequences because of the relatively high lipid content. A ratio between nerve fascicles and perifascicular tissue can be calculated using software and is referred to as FR. The rationale of this quantitative evaluation is that the ratio between fascicular tissue and nonfascicular tissue changes under different pathological conditions.10,11 It has been already demonstrated that, using US, the FR is capable of discriminating between normal and pathological nerves of patients affected by carpal tunnel syndrome or neurofibromas. In addition, the FR can discriminate between patients with mild and severe carpal tunnel syndrome.10 Evaluation of the FR may also be useful in conditions in which the nerve size is not affected, and the only change is in the ratio between fascicles and perifascicular tissue. One example could be chronic atrophy with loss of nerve fascicles and an increase in perifascicular tissue. FR assessment is a promising step toward advancing the US armamentarium of peripheral nerve assessment. Similarly, FR assessment on MRI could be a new step in evaluating the peripheral nervous system. The FR evaluated on US is thought to be reliable in entrapment neuropathies,10,11,15 and its role in other neuropathies is under evaluation. Therefore, the purpose of this study was to define and quantitatively evaluate FR on MRI in peripheral neuropathies compared with healthy controls and to assess if MRI has the potential to detect FR changes in neuropathy. In addition, we assessed if FR values obtained on US correlate with those obtained on MRI.
MATERIAL AND METHODS
Written and verbal consent was obtained from all patients and healthy volunteers. The study protocol was completed entirely on anonimized images and approved by the Ethics Committee (181REG2013).
Patients undergoing a clinical MRI study of the extremities for any indication (eg, musculoskeletal or neurography) were included if they consented. Overall, a group of 40 control patients with no neuropathy, including patients who underwent MRI for musculoskeletal pain of nonneurological origin, (20 women, 20 men; age, 44.6 ± 13.4 years) and 40 patients with peripheral neuropathy referred by the Neurological Department of IRCCS AOU San Martino (Genova, Italy; 22 women, 18 men; age, 50.3 ± 10.2 years), were examined with a standard 3T MRI protocol and high-resolution US for correlation. Patients who underwent examination of an extremity for indications other than clinically apparent neuropathy were considered healthy controls for FR evaluation. In addition, controls with risk factors for neuropathy, such as diabetes, alcoholism, and metabolic or infectious diseases, were excluded. All patients had electrophysiologically confirmed or suspected peripheral nerve pathology. All patients had the clinically characteristic symptoms and the results of their electrodiagnostic tests were obtained within 2 weeks before the MRI and US examinations. Patients were further classified according to the etiology of the disease as entrapment neuropathy, inflammatory neuropathy, traumatic nerve injury, hereditary polyneuropathy, or, if clinical, electrophysiological, and imaging examinations had been inconclusive as undetermined cause. Entrapment neuropathy was diagnosed if a patient presented with symptoms in the distribution of 1 nerve compatible with peroneal tunnel entrapment at the fibular head, or tarsal or radial tunnel entrapment. No patients with carpal tunnel syndrome or ulnar neuropathy at the elbow were included because these neuropathies are not evaluated with MRI at our center. In addition, for carpal tunnel syndrome or ulnar neuropathy at the elbow, MRI is unlikely to give additional information over US as stated by the guidelines of the European Society of Musculoskeletal Radiology.16 Patients were classified as inflammatory neuropathy if a polyneuropathy was supported by clinical, laboratory, and electrophysiological examinations or if a mononeuropathy outside an osteofibrous tunnel was diagnosed, such as multifocal motor neuropathy or chronic inflammatory demyelinating polyneuropathy (CIDP). If the underlying etiology was unknown after clinical, laboratory, and electrophysiological tests, patients were classified as disseminated neuropathy of undetermined cause. If a hereditary cause for the neuropathy was known, such as hereditary sensory and motor neuropathy or Charcot–Marie–Tooth disease, the patients were classified as hereditary polyneuropathy, and finally cases with known history of blunt or sharp trauma to the nerve were classified as traumatic nerve injury.
We used customized software that had been adapted from previous software used to calculate the FR on US images. The hypointense and hyperintense areas of the peripheral nerves were examined on T1-weighted MRI sequences, because on these sequences we observed better differentiation between fascicular and nonfascicular tissue. The ratio of hypointense pixels/total pixels on MRI images was called FR and was recorded.
MRI Protocol
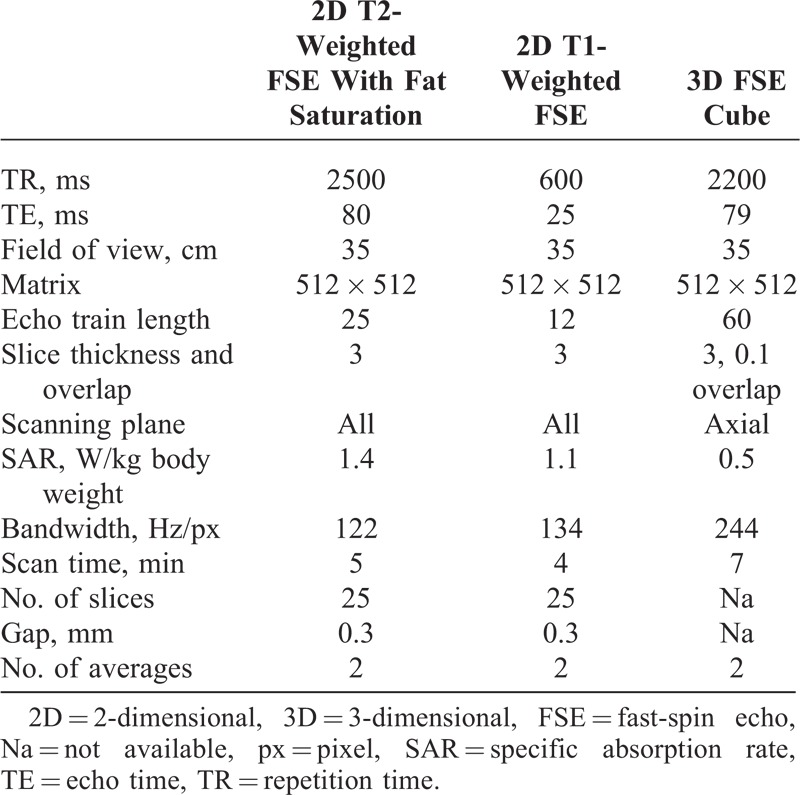
MRI examinations were performed at a 3T magnetic field strength (GE Signa HDx 3.0T; General Electric Medical Systems, Milwaukee, WI) between January 2012 and July 2013. The 3T MRI system with the employed extremity coil provided optimal signal to visualize fascicles within the nerve. At lower field strength, such detail may not be achievable.12 To study the peripheral nerves, the following sequences were acquired: T2-weighted turbo-spin-echo sequence with spectral fat saturation and at high spatial resolution for reliable recognition and differentiation of peripheral nerve fascicles. The slab position of this first sequence was tailored to the patient’s known or most likely location of maximum nerve lesion as stated by clinical and electrophysiological findings. Then, T1-weighted turbo-spin-echo sequences with and without spectral fat saturation and a 3-dimensional (3D) fast-spin echo (FSE) cube sequence were added. 3D FSE-cube sequences were added if it was necessary to visualize pertinent 3D anatomy for diagnostic purposes. Sequence parameters are reported in Table 1.
TABLE 1.
Parameters of MRI Sequences
A knee 8-channel transmit/receive phased array radiofrequency coil was used in 38 subjects, and a cardiac phased array coil 8-channel receiver radiofrequency coil was used in 22 subjects.
US Technique
A commercially available machine (iU22; Philips Medical Systems, Eindhoven, The Netherlands) equipped with broadband linear array transducers (frequency band, 5–17 MHz) was used to study the nerves in patients and control subjects to correlate the FR obtained with MRI to that obtained with US. All US studies were performed by the same musculoskeletal radiologist (A.S.T.) with 8 years of experience in musculoskeletal imaging, and all of the images were obtained on planes perpendicular to the main axis of the nerve. Compound imaging was used to reduce angle-generated and speckle noise artifacts and to better visualize curved and irregular borders.10
The focal depth and the overall gain adjustment was set to obtain the best scan possible and adjusted at every level to obtain the best images. For US image analysis, the semiautomated method was used because it has been shown to be more reliable.10 The images were recorded for every nerve, and at least 8 images for a single nerve were included. The mean values were registered for the final analysis. FR calculated on US was defined as the ratio between the hypoechoic and hyperechoic areas of peripheral nerves. The FR was defined as the ratio of hypoechoic pixels/total pixels of the nerve visualized on US, and it was calculated as previously reported.10
Image Analysis With the Semiautomated Software
On MRI, all peripheral nerves at all slice positions (median, ulnar, and radial nerves for upper arm examinations; sciatic nerve for thigh; peroneal and tibial nerve for lower limb examinations) were evaluated and a mean value was calculated from all individual slice measurements for each nerve. The extreme positions were not included because of potential aliasing artifacts of the imaging slab. Precise regions of interest (ROIs) were drawn by an expert user around the epineurial contour of peripheral nerves in the axial sequences using T1-weighted images as a reference with the OsiriX software v3.9.4 (www.osirix-viewer.com). They were subsequently analyzed with our customized software. Images corresponding to nerve segmentations were stored as 256 × 256 images, and a 181.11 px/cm resolution was considered sufficient for analysis. Through a user-guided interactive procedure, images were visually analyzed separating the fascicular tissue (hypointense on T1-weighted sequences) from the nonfascicular tissue (hyperintense on T1-weighted sequences without fat saturation). Based on this pixel classification, procedure pixels are separated into the following 2 classes: pixels above the selected intensity threshold and pixels below the threshold (Figure 1). The FR was then calculated by dividing the number of fascicular pixels below the threshold by the total number of pixels in the entire nerve region. The software used in this study (MedDensity; G.T. and A.S.T.) uses an existing image processing method for estimating FR10 on US. On every image, the threshold was visually set by a radiologist who had experience in peripheral nerve imaging and by an engineer who had developed the software (A.S.T., 7 years of experience; G.T., 5 years of experience), to assure optimal separation between fascicles and perifascicular tissue and to minimize artifacts related to image segmentation and quality. This manual thresholding process is commonly employed in clinical practice for imaging breast tissue17 and muscle.18 The image processing was developed based on previous research.17–20 The digital images obtained were analyzed by 3 readers, who were blinded to each other and who had completed appropriate training with this method (A.S.T., 7 years of experience; G.T., 5 years of experience; another researcher with 3 years of experience in quantitative image analysis). After the first evaluation, measurements were repeated 2 months later to assess intraobserver variability.
FIGURE 1.
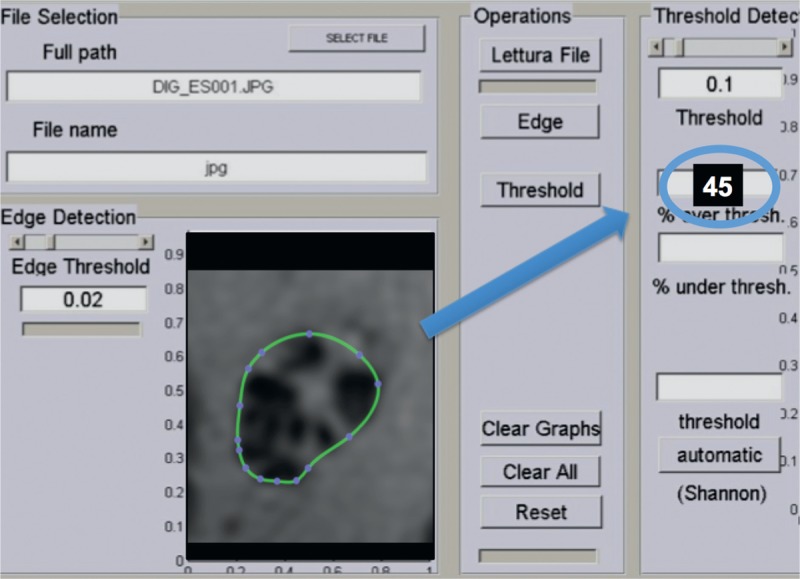
Quantitative assessment of the FR with the semiautomatic or interactive method. In this figure, the computer interface is shown. The nerve is identified within a region of interest. In the semiautomatic version, the radiologist adjusts the density threshold to separate fascicle and nonfascicles. The FR is read into the blue circle. In the fully automated method, there is no need to adjust the threshold. In this figure, artificially modified for visual purposes, the FR is 45%. FR = fascicular ratio.
Image Analysis With the Automated Software
The automatic evaluation of the FR is based on the same images of the semiautomatic process. The same ROIs are used as input to the automatic evaluation. To obtain the required pixel classification, a histogram-based analysis is performed, which was adapted from a previous application.17 For this application, a maximum entropy thresholding criteria was used, which was able to identify a pixel intensity value that divides dense from nondense pixels.17 The FR is computed as the ratio between pixels below the threshold and the total number of pixels.
The time to perform both automatic and semiautomatic evaluations was calculated by a fellow not involved in the study, using a commercially available stopwatch.
Statistical Analysis
Statistical analysis was performed using the Mann–Whitney U test for unpaired data to compare patients and healthy controls. Values were expressed as the mean ± standard deviation. Probability (P) values <0.05 were considered statistically significant.
P values were adjusted for multiple comparisons using the Bonferroni–Holm correction. Statistical evaluation was performed using a commercially available software package (SPSS, release 13.0 for Windows). A post hoc power analysis was performed to ensure that our sample size was sufficient for a meaningful statement. An α error level or confidence level of 5% and a β error level or statistical power (1–β) of 80% was used and considered acceptable for medical purposes. A sample size of 15 enabled confidence within the required confidence ranges. The power analysis was performed using a commercially available software package (Power and Precision V3; Biostat). Receiver operating characteristic (ROC) analysis was performed to determine the value of the FR as a marker of pathology. Intraobserver and interobserver agreements between the 3 readers in the evaluation of the FR were determined with κ. The κ interobserver variability was used to measure the degree of agreement, and <0.21, 0.21 to 0.4, 0.41 to 0.6, 0.61 to 0.8, and 0.81 to 1 were considered poor, fair, moderate, good, and very good agreements, respectively.21 Correlation between the FR obtained on MRI and US was determined with the Pearson test and linear regression analysis. In addition, the correlation between the FR and the T2 signal intensity and nerve cross-sectional area was obtained as well.
RESULTS
Forty control subjects (20 women, 20 men; age, 44.6 ± 13.4 years) and 40 patients with peripheral neuropathy (22 women, 18 men; age, 50.3 ± 10.2 years) based on clinical and electrophysiological results were studied.
Patients were further classified by etiology into 22 patients with an entrapment neuropathy, 8 patients with an inflammatory neuropathy, 4 patients with traumatic nerve injury, 4 patients with a known hereditary polyneuropathy, and 2 patients with a polyneuropathy of yet undetermined cause. Patients affected by tumors of the peripheral nervous system were excluded from the study.
By body region, examinations covered the upper limb in 18 control subjects and 16 patients, including the arm in 3 and 2 subjects, respectively, the elbow in 9 and 6 subjects, respectively, and the wrist in 6 and 8 subjects, respectively. Examinations covered the lower limb in 22 controls and 24 patients, including the thigh in 8 and 7 subjects, respectively, the knee in 6 and 8, subjects, respectively, and the leg in 8 and 9 subjects, respectively.
Program Performance and Time
None of the images were excluded from the evaluation because of technical problems, and it was always possible to calculate FR. A very brief training was needed to learn the software interface and all of the features of the tool. The learning curve for this program was very steep, and the radiologists involved in this study were able to complete their evaluations in <2 minutes (mean time, 102 seconds).
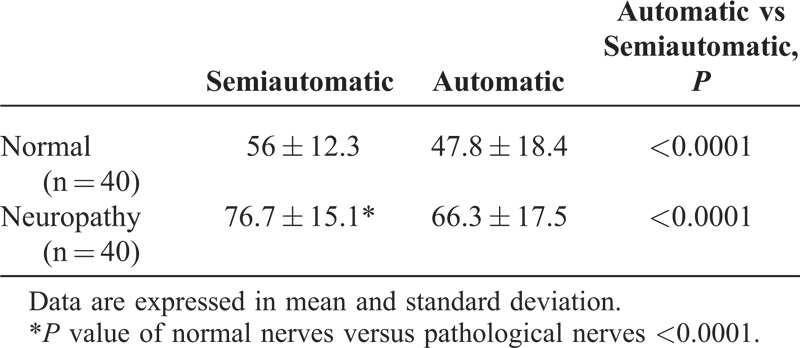
Overall quantitative analysis of the FR showed that patients with neuropathy had a significantly higher FR than control subjects (Table 2) with both interactive and automatic evaluations.
TABLE 2.
Data Regarding Nerve Density Quantitative Analysis Using the Semiautomatic and the Automatic Software
Subgroup analyses for neuropathies with different etiologies showed that the FR was significantly higher in traumatic nerve injury (Figure 2) than in any other group (Table 3). The FR values in entrapment neuropathies were found to overlap with control subjects and neuropathies of other etiologies.
FIGURE 2.
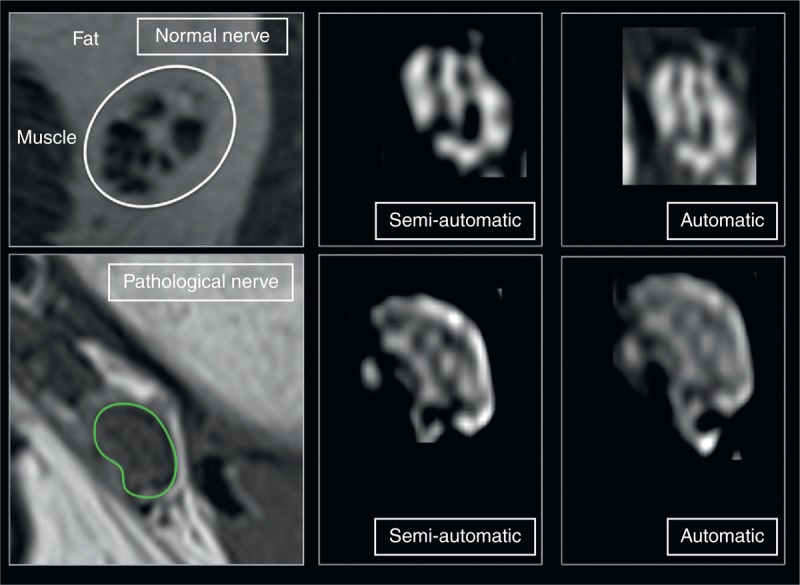
Quantitative analysis of FR. Regions of interest were drawn around the epineurial contour of peripheral nerves in axial T1-weighted images (green line). Quantitative analysis was performed on every slice, extremes excluded. On MRI images, the software, after threshold identification, differentiated the fascicles (hypointense on T1-weighted sequences) and the perifascicular tissue (hyperintense on T1-weighted sequences) that resembles the signal of fat. The FR was calculated based on the volume of the fascicles in relation to the total volume of the nerve. The pathological nerve is a tibial nerve. The results are reported in the text and tables. FR = fascicular ratio, MRI = magnetic resonance imaging.
TABLE 3.
Data Regarding the Comparison Between Different Etiologies Using the Mann–Whitney U Test for the Semiautomatic Method
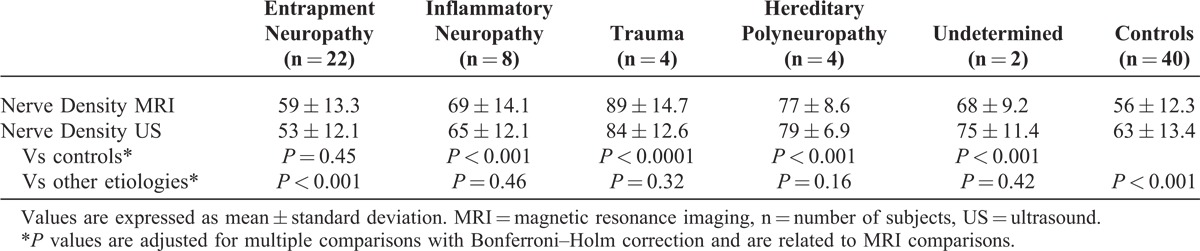
The ROC analysis was performed to assess the potential utility of the FR as a diagnostic marker for peripheral neuropathies. The FR had good accuracy for the detection of peripheral neuropathies. Exclusion of entrapment neuropathies improved the diagnostic accuracy because they had overlapping values with control subjects (ROC values are given in Table 4).
TABLE 4.
ROC Analysis of Diagnostic Performance for FR
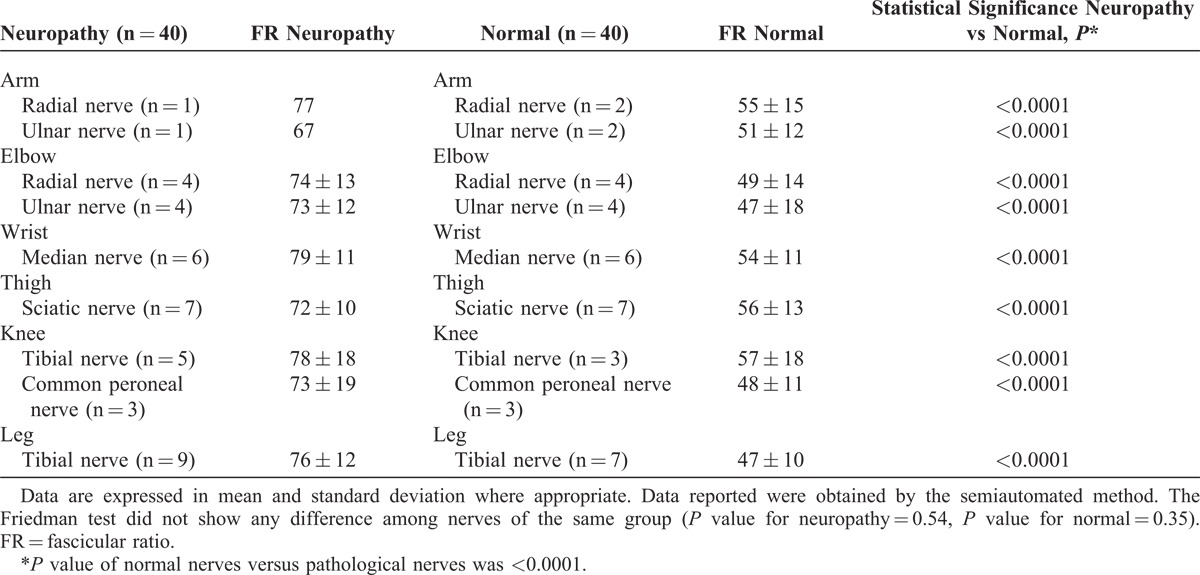
The FR values were independent of the body region in which they were assessed in healthy control subjects and patients (Table 5).
TABLE 5.
Data Regarding FR Quantitative Analysis for Single Nerves
No significant correlation between age and FR was observed.
A good correlation was observed between the FR calculated on MRI and US (r = 0.49; 95% confidence interval [CI] for r, 0.21–0.70; P = 0.012).
A positive correlation was observed between the FR calculated on MRI and T2 signal intensity (r = 0.29; 95% CI for r, 0.11–0.44; P = 0.03).
A lack of correlation was observed between the FR calculated on MRI and the nerve cross-sectional area (r = 0.05, P = 0.65).
Intraobserver Agreement
Using the semiautomated software, the average-weighted intraobserver agreement of the 3 readers 2 months after the first evaluation was good K = 0.86.
Interobserver Agreement
Interobserver agreements among the 3 readers in the evaluation of the FR were considered to be good as well, based on the following results: reader 1 versus reader 2, k = 0.71 (95% CI, 0.64–0.90); reader 2 versus reader 3, k = 0.78 (95% CI, 0.74–0.90); reader 3 versus reader 1, k = 0.71 (95% CI, 0.65–0.81).
FR Analysis With Fully Automated Software
Using the fully automated software there was no need for intraobserver and interobserver agreement evaluation, because the software provides the same result for the same image. Data regarding FR evaluated with both methods are reported in Table 2.
Differences Between the Automated and the Semiautomated Analysis
As shown in Table 2, there is a significant difference between the automated and the semiautomated software due to the fixed threshold that the automatic software has to use.
DISCUSSION
Quantitative assessment of nerve echogenicity or the FR has been considered a step further in the evaluation of peripheral nerves by the means of US.11,15 Some authors evaluated 1 manual and 16 automatic thresholding methods on US for quantitative nerve echogenicity assessment by comparing 56 patients with ulnar neuropathy at the elbow and 37 healthy control subjects, and they found a significant difference in the mean hypoechogenic fraction between patients with ulnar neuropathy and control subjects in different automatic thresholding methods, showing that quantitative nerve echogenicity assessment can be helpful in distinguishing between ulnar neuropathy and healthy tissue.15 Another study showed that differences in the median FR differentiate between normal median nerves, neurofibroma, and carpal tunnel syndrome.10 Other authors used a quantitative method (ImageJ software; National Institutes of Health, Bethesda, MD) and showed that compared with healthy subjects hypoechogenicity of the median nerve was increased in diabetic neuropathy.22 Quantitative nerve echogenicity/density assessment was considered a step toward advancing the US potential in peripheral nerve assessment.11 Reflecting the studies based on US, we believe that the FR could also be evaluated on MRI. In this study, we described a feasible technique to estimate the alteration in the fascicular pattern of the peripheral nerves. The study of FR on MRI resulted in a feasible method to differentiate neuropathy from normal nerves in the majority of cases. This new measurement may be added to the standard evaluations made on MRI and may provide new insights on the study of peripheral nerves on MRI. Although FR indicates neuropathy, according to our data, this statement may be true not only for acute neuropathy but also for long standing diseases with loss of nerve fascicles and an increase in perifascicular fat. An example could be inflammatory and autoimmune neuritis of different etiologies, such as CIDP and scleroderma with chronic inflammatory damage to the peripheral nerve.23 In addition, the quantification of nerve fibers could be used in adjunct and to support histology. For example, nerve fibers were quantified histologically to enhance regeneration.24 It has been shown that the density of fibers was different depending on the treatments applied. The concept of FR evaluated with US and MRI could be considered an extension and an adjunct to histology with no need for nerve biopsy. For nerve regeneration, we believe that FR could be used to quantify the density of fibers as it is done with histology. The FR was increased in neuropathies with different etiologies. The reason may be that nerve fascicles increase in caliber because of intraneural edema and inflammation, whereas the epineurial tissue is only slightly involved by the pathological process. Therefore, the ratio between hypointense fascicles on T1-weighted sequences and the total amount of tissue of the nerve changes accordingly. In our series, the FR values in entrapment neuropathies overlapped with those from control subjects. These data may be considered counterintuitive because in entrapment neuropathies the fascicular structure of the nerve is usually altered. However, in our centers, MRI is usually only performed in cases in which US in not straightforward. It is likely that the patients who underwent MRI at our center had only mild or moderate neuropathies; therefore, it is possible that the FR was not altered because of fascicular edema that was too small to be detected by the software. We believe that some common mechanisms altering the fascicles and the blood–nerve permeability may have influenced the different MRI parameters related to the inner structure of the nerve, such as T2 signal intensity, perfusion, and diffusion.1 Indeed, we found a weak correlation between T2 signal intensity and FR but not with the cross-sectional area. These data support the hypothesis that the FR could be considered an independent indicator of peripheral neuropathy.
In spite of the positive results of the present study, there are several issues that remain unresolved due to the preliminary nature of the research. We were not able to assess which anatomical area of the nerve is the most appropriate for FR analysis. We believe that, hypothetically, the most appropriate area should be the one where a previous US study, if available, had demonstrated an alteration of the normal fascicular pattern. The lack of a significant correlation between age and FR may be worth further investigations, because it is possible that 40 patients were not sufficient to demonstrate very slight changes in peripheral nerves due to normal aging. The strong correlation between the FRs calculated on MRI and US is a sign of reliability of the method used to estimate the FR on MRI. More sophisticated comparisons between US and MRI for FR assessment are beyond the purpose of this study and warrant further investigations. Regarding the reliability of US measurements of FR, the intraobserver agreement was found to be good as was the interobserver agreement. In addition, it was possible to assess FR on US using a fully automated software with no need of intraobserver and interobserver agreement.10
An important issue was the quality of the produced images, which was dependent on the technical hardware and software used to produce the MRI images to be evaluated. In this study, a high-field 3T MRI was used. We acknowledge that potentially large variations between different MRI machines are possible. In addition, clinical 1.5T MRI systems may not be able to produce peripheral nerve images suitable for FR evaluation. Despite these limitations, quantitative evaluation of FR reflecting the inner structure of the peripheral nerve seems feasible and able to differentiate normal from pathological nerves in the majority of cases as demonstrated by the ROC analysis. The diagnostic accuracy calculated by the ROC analysis may be considered comparable with the diagnostic accuracy of diffusion tensor imaging and perfusion parameters.1 The difference between the automated and the semiautomated analysis could be explained because the automated software has a fixed threshold that has to be chosen before the analysis. The semiautomated software has the advantage that radiologists or researchers can select the best threshold for differentiating fascicles from nonfascicles. This process should reduce overlaps between tissues with similar values. The differences between semiautomated and fully automated software packages have already been observed in breast imaging.17
To our knowledge, this is the first study investigating the FR in vivo by MRI using quantitative evaluation.
Further studies are needed to determine the clinical usefulness of the FR and to assess its relationship with clinical, electrodiagnostic, and US parameters as well as clinical outcome.
In conclusion, we have presented a method for quantifying the inner structure of peripheral nervous tissue based on the relationship between fascicular and nonfascicular tissue. The feasibility of the technique is supported by data in healthy control subjects and patients with neuropathies. Quantitative evaluation of the FR may be used in future investigations on peripheral neuropathy.
Footnotes
Abbreviations: 3D = 3-dimensional, CI = confidence interval, CIDP = chronic inflammatory demyelinating polyneuropathy, FR = fascicular ratio, FSE = fast-spin echo, MRI = magnetic resonance imaging, ROC = receiver operating characteristic, US = ultrasound.
The study was partially funded by the University of Genova (PRA 2012-PRA 2013).
The authors have no conflicts of interest to disclose.
References
- 1.Bäumer P, Reimann M, Decker C, et al. Peripheral nerve perfusion by dynamic contrast-enhanced magnetic resonance imaging: demonstration of feasibility. Invest Radiol. 2014;49:518–523. [DOI] [PubMed] [Google Scholar]
- 2.Bäumer P, Dombert T, Staub F, et al. Ulnar neuropathy at the elbow: MR neurography–nerve T2 signal increase and caliber. Radiology. 2011;260:199–206. [DOI] [PubMed] [Google Scholar]
- 3.Chalian M, Soldatos T, Faridian-Aragh N, et al. 3T magnetic resonance neurography of tibial nerve pathologies. J Neuroimaging. 2013;23:296–310. [DOI] [PubMed] [Google Scholar]
- 4.Tagliafico A, Succio G, Serafini G, et al. Diagnostic accuracy of MRI in adults with suspect brachial plexus lesions: a multicentre retrospective study with surgical findings and clinical follow-up as reference standard. Eur J Radiol. 2012;81:2666–2672. [DOI] [PubMed] [Google Scholar]
- 5.Beeckman R, Schoemaker MC, Van der Plas JP, et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62:767–773. [DOI] [PubMed] [Google Scholar]
- 6.Padua L, Aprile I, Pazzaglia C, et al. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: a one-year systematic assessment. J Clin Neurophysiol. 2007;118:1410–1416. [DOI] [PubMed] [Google Scholar]
- 7.Tagliafico A, Bignotti B, Miguel Perez M, et al. Contribution of ultrasound in the assessment of patients with suspect idiopathic pudendal nerve disease. Clin Neurophysiol. 2014;125:1278–1284. [DOI] [PubMed] [Google Scholar]
- 8.Martinoli C, Miguel-Perez M, Padua L, et al. Imaging of neuropathies about the hip. Eur J Radiol. 2013;82:17–26. [DOI] [PubMed] [Google Scholar]
- 9.Martinoli C, Tagliafico A, Bianchi S, et al. Peripheral nerve abnormalities. Ultrasound Clin. 2007;2:655–667. [Google Scholar]
- 10.Tagliafico A, Tagliafico G, Martinoli C. Nerve density: a new parameter to evaluate peripheral nerve pathology on ultrasound. Preliminary study. Ultrasound Med Biol. 2010;36:1588–1593. [DOI] [PubMed] [Google Scholar]
- 11.Wilder-Smith EP. Quantitative assessment of peripheral nerve ultrasound echogenicity. A step forward. Clin Neurophysiol. 2012;123:1267–1268. [DOI] [PubMed] [Google Scholar]
- 12.Pham M, Bäumer P, Meinck HM, et al. Anterior interosseous nerve syndrome: fascicular motor lesions of median nerve trunk. Neurology. 2014;82:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husarik DB, Saupe N, Pfirrmann CW, et al. Elbow nerves: MR findings in 60 asymptomatic subjects–normal anatomy, variants, and pitfalls. Radiology. 2009;252:148–156. [DOI] [PubMed] [Google Scholar]
- 14.Subhawong TK, Wang KC, Thawait SK, et al. High resolution imaging of tunnels by magnetic resonance neurography. Skeletal Radiol. 2012;41:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boom J, Visser LH. Quantitative assessment of nerve echogenicity: comparison of methods for evaluating nerve echogenicity in ulnar neuropathy at the elbow. Clin Neurophysiol. 2012;123:1446–1453. [DOI] [PubMed] [Google Scholar]
- 16.Klauser AS, Tagliafico A, Allen GM, et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol. 2012;22:1140–1148. [DOI] [PubMed] [Google Scholar]
- 17.Tagliafico A, Tagliafico G, Tosto S, et al. Mammographic density estimation: comparison among BI-RADS categories, a semi-automated software and a fully automated one. Breast. 2009;18:35–40. [DOI] [PubMed] [Google Scholar]
- 18.Tagliafico AS, Ameri P, Bovio M, et al. Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol. 2010;194:728–734. [DOI] [PubMed] [Google Scholar]
- 19.Wong AKC. A gray-level threshold selection method based on maximum entropy principle. IEEE Trans Syst Man Cybernet. 1989;19:866–871. [Google Scholar]
- 20.Zhou C, Chan HP, Petrick N, et al. Computerized image analysis: estimation of breast density on mammograms. Med Phys. 2001;28:1056–1069. [DOI] [PubMed] [Google Scholar]
- 21.Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–308. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Ito H, Sekine A, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697–708. [DOI] [PubMed] [Google Scholar]
- 23.Tagliafico A, Panico N, Resmini E, et al. The role of ultrasound imaging in the evaluation of peripheral nerve in systemic sclerosis (scleroderma). Eur J Radiol. 2011;77:377–382. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X1, Zhang F, Lu L, et al. MR imaging and T2 measurements in peripheral nerve repair with activation of Toll-like receptor 4 of neurotmesis. Eur Radiol. 2014;24:1145–1152. [DOI] [PubMed] [Google Scholar]


