Abstract
Many studies have reported the prognostic value of pretreatment serum carcinoembryonic antigen (pre-CEA) levels on colorectal cancer outcomes. However, controversy remains concerning the significance of pre-CEA levels in patients with rectal cancer treated with neoadjuvant chemoradiotherapy (CRT). Our aim in this study was to investigate the prognostic role of the pre-CEA level in patients with locally advanced rectal cancer undergoing neoadjuvant CRT followed by total mesorectal excision (TME).
A total of 419 patients with stages II and III rectal cancer treated with neoadjuvant CRT followed by TME with available pre-CEA data were included. The outcomes studied were 5-year local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and disease-free survival (DFS). Optimal pre-CEA cutoff values to predict DMFS were determined based on current smoking history.
The median pre-CEA level of smokers was 3.8 ng/mL, and that of nonsmokers was 2.8 ng/mL (P < 0.01). Pre-CEA levels of 6.6 ng/mL for nonsmokers and 11.4 ng/mL for smokers were determined to best separate patients on the basis of time to distant metastasis by using log-rank statistics. The pre-CEA level was associated with DMFS (hazard ratio = 1.743, 95% confidence interval = 1.129–2.690, P = 0.01). The pre-CEA level was not associated with LRFS or DFS.The pre-CEA level appears to be a significant preoperative prognostic factor. Moreover, it is as valuable as any known pathologic factor. Future studies evaluating oncologic outcomes should take into consideration the pre-CEA level.
INTRODUCTION
Preoperative chemoradiotherapy (CRT)1–3 and total mesorectal excision (TME)4,5 are widely accepted as the treatment of choice for locally advanced distal rectal cancer, and this multidisciplinary approach has dramatically improved local control from an unacceptable local recurrence rate of 25% to 40% to <10%.1,2,4–6 However, 25% to 40% of patients still die of metastatic disease.2,3,7,8 Although survival depends primarily on distant metastasis, the treatment of advanced rectal cancer has focused on reducing local recurrence. Even neoadjuvant chemotherapy is intended as a radiosensitizer to reduce local recurrence rather than prevent systemic metastasis.
Therefore, to improve the survival of patients with advanced rectal cancer, reduction of distant metastasis is needed. An accurate predictor of distant metastasis could greatly benefit to select high-risk patients, especially before treatment initiation. The Union for International Cancer Control tumor–node–metastasis (TNM) classification is regarded as the best predictor of oncologic outcome. In addition to TNM staging, the College of American Pathologists identified 4 classes of colorectal prognostic markers; class I includes blood and lymphatic vessel invasion, residual tumor, and preoperative serum carcinoembryonic antigen (pre-CEA) level.9 Of these markers, reappraising the prognostic role of pre-CEA in rectal cancer treated with neoadjuvant CRT is important for several reasons. First, serum CEA is the only marker that yields presurgical information. Second, accurately assessing the anatomical extent of rectal cancer at the time of diagnosis is currently limited because of imprecise imaging tools,10,11 in particular, the assessment of nodal stage, the strongest predictor of outcome,11 is challenging. Finally, even the postneoadjuvant CRT pathologic stage (yp stage) cannot represent the initial metastatic burden because of variable downstaging, and subsequently, cannot provide differentiated adjuvant treatment strategies. The National Comprehensive Cancer Network guidelines suggest that adjuvant chemotherapy be administered for all patients treated with neoadjuvant CRT, regardless of pathologic stage, even following an apparent complete response.12
Several studies have shown that elevated pre-CEA was an independent poor prognostic factor in colorectal cancer.13–17 Moreover, a recent large study suggested that CEA level (C-stage) be included in the conventional TNM staging of colon cancer.18 However, there are substantial clinical barriers to evaluating CEA as a prognostic factor, including the variable definition of elevated CEA, heterogeneous study cohorts, and factors associated with elevated CEA, such as other neoplastic and nonneoplastic conditions.19–21 Therefore, in this study, we determined pre-CEA cutoff values and evaluated the efficacy of pre-CEA as a predictive factor for distant metastasis in patients treated with neoadjuvant CRT.
METHODS
Patients
Between February 2005 and December 2012, 419 consecutive patients who received neoadjuvant CRT for locally advanced (radiologic T3-T4 or N+ and/or clinically bulky) mid-to-low rectal cancer were included in this prospective study. Only patients who completed full-course neoadjuvant CRT were included. Exclusion criteria included local excision after neoadjuvant CRT, unavailable pre-CEA values, concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, other malignancy, and newly developed distant metastasis during neoadjuvant CRT. The smokers group was defined as patients who smoked at the time of rectal cancer diagnosis. The nonsmokers group included both ex-smokers (n = 40), who had stopped smoking at least 6 months previously (n = 37) or before the diagnosis of rectal cancer (n = 3), and patients who had never smoked. Two patients who had stopped smoking after the diagnosis of rectal cancer were included in the current smoker group. This study was performed with approval of the institutional review board of Chonnam National University Hwasun Hospital, Gwangju, South Korea.
Staging and Treatment
Staging: The preoperative clinical stage of most patients (396, 94.5%) was determined by rectal magnetic resonance image (MRI). Abdominopelvic computed scan (CT) and endorectal ultrasound were used for staging in 16 (3.8%) and 7 (1.7%) patients, respectively.
Neoadjuvant CRT: All patients received 5-fluorouracil (5-FU)-based chemotherapy with concomitant external beam radiation using a 4-field box technique preoperatively. During weeks 1 and 5 of radiotherapy, 5-FU (425 mg/m2/d) and leucovorin (20 mg/m2/d) were administered intravenously.
Surgery: At 6 to 8 weeks following completion of neoadjuvant CRT, surgery was performed based on the same oncologic policy among surgeons.22
Postoperative adjuvant chemotherapy: After recovery from surgery, all patients who underwent radical surgery were considered for postoperative chemotherapy.
Pathology and Follow-Up
All resected specimens were examined by 2 gastrointestinal pathologists, and tumor regression induced by neoadjuvant CRT was defined as the ratio of fibrosis to residual viable tumor. The detailed tumor regression grade (TRG) scores were: TRG1 (<25% fibrosis), TRG2 (25%–50% fibrosis), TRG3 (50%–75% fibrosis), and TRG4 (>75% fibrosis). Pathologic complete response (pCR) was defined as the absence of viable tumor cells with no lymph node involvement.23,24 Patients underwent standardized follow-up at 3-month intervals for 2 years and 6-month intervals thereafter for a total of 5 years. Follow-up included physical examination, complete blood count, blood chemistry tests, and serum CEA assay. Distant metastasis was detected by abdominopelvic and chest CTs. Neither rectal MRI nor positron emission tomography/CT scan was used routinely.
Statistical Analysis
The χ2 test or Fisher exact test was used to analyze categorical variables, and Student t test and the Wilcoxon rank-sum test were used for continuous variables. Univariable analysis was performed to evaluate the prognostic impact of pre-CEA level on the local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and disease-free survival (DFS). The maximal χ2 method was adapted to determine which pre-CEA value best separated patients into poor- and good-prognosis subgroup (in terms of the likelihood of surviving), and the log-rank test was used to measure the power of the grouping. The R MaxStat package (R Foundation for Statistical Computing, Vienna, Austria) was used for this analysis.25 Analysis according to smoking status was performed. The Kaplan–Meier method was used to establish the effects of each variable, and log-rank tests were used to compare survival curves. Multivariate analyses of survival were conducted using Cox proportional hazards models. Significant variables in univariate analysis (P < 0.1) were entered into regression models with increasing complexity, and significance was assessed using analysis of variance analysis. The performance of predictive models was compared by the likelihood-ratio test, using the “coxph” and “anova” functions, which are included in the R “survival” package. Results with a P value < 0.05 were considered significant. Statistical analysis was performed using R statistical software, version 3.1.1.
RESULTS
Patient Characteristics and Association Between Pre-CEA Level and Clinicopathologic Factors
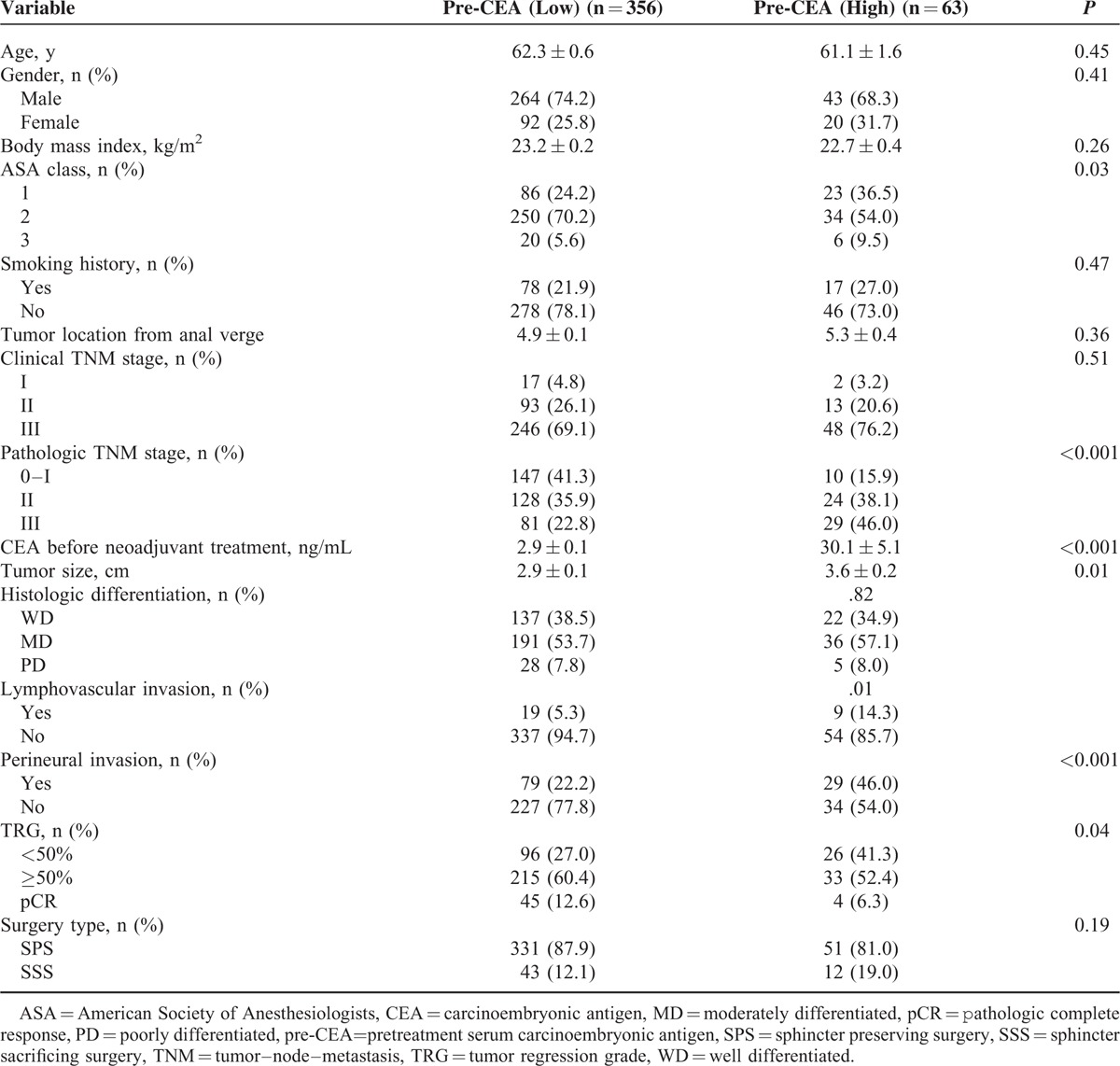
Of 419 patients, 307 (73.3%) were men. The mean age was 64.1 years (range, 27–87 years). A total of 364 (86.9%) patients underwent anterior resection, 51 (12.2%) underwent abdominoperineal resection, and 4 (0.9%) underwent Hartmann procedure. The median pre-CEA level was 3.0 ng/mL (interquartile range, 1.9–4.9 ng/mL). The median pre-CEA level of smokers was 3.8 ng/mL, and that of nonsmokers was 2.8 ng/mL (P < .01). The pre-CEA levels of 6.6 ng/mL for nonsmokers and 11.4 ng/mL for smokers were determined to best separate patients on the basis of time to distant metastasis (Figure 1). Based on these cutoff values, 46 (12.2%) nonsmokers and 17 (17.9%) smokers were classified as the high-CEA group. Clinical and pathologic features according to pre-CEA level are shown in Table 1. Patients in the high-CEA group were significantly more likely to have a more aggressive pathologic stage and poor prognosis. Higher pathologic tumor stage, larger pathologic tumor size, lymphovascular invasion, and perineural invasion were significantly higher in the high-CEA group than in the low-CEA group. Moreover, poor radiation response (TRG 1+2) was significantly more frequent in the high-CEA group than in the low-CEA group.
FIGURE 1.
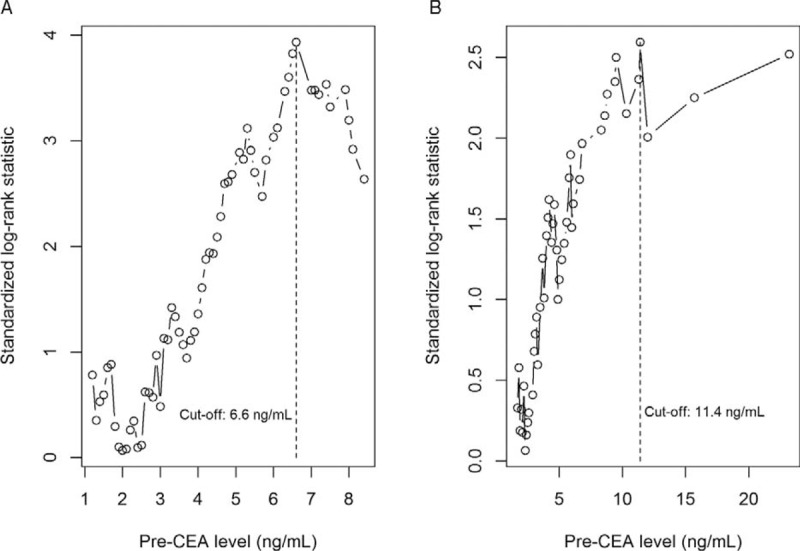
Cutoff value of pre-CEA. Nonsmoker group were ranked according to increased pre-CEA level and a maximum difference in distant metastasis-free survival was determined with pre-CEA level = 6.6 ng/mL in nonsmoker group (A) and 11.4 ng/mL in smoker group (B). pre-CEA=pretreatment serum carcinoembryonic antigen.
TABLE 1.
Clinical and Pathologic Characteristics According to Preoperative Level of Cancinoembryonic Antigen
Survival
In total, 368 surviving patients underwent follow-up for a median 42.2 months (interquartile range, 27.6–55.5 months). Thirty-nine (9.3%) patients had local recurrence. Twenty-nine had local recurrence alone, and 10 had synchronous distant metastasis. Distant metastasis occurred in 109 (26.0%) patients, primarily involving the lung (61.5%) and liver (34.8%).
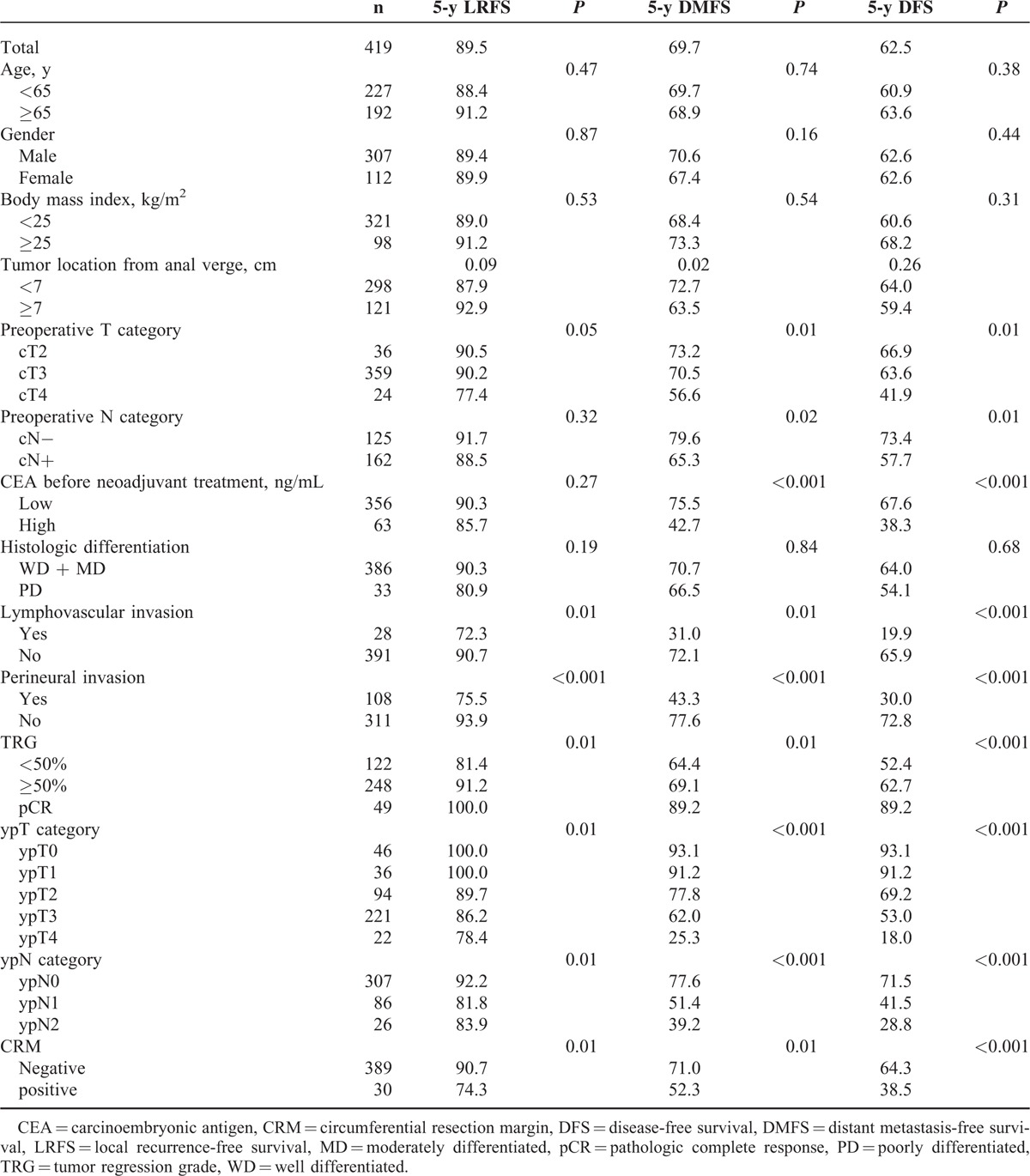
The pre-CEA was not a prognostic factor for 5-year LRFS, but was significantly associated with 5-year DMFS (Figure 2). Indeed, the cumulative incidence of distant metastasis was 21.9% and 49.2% in the low and high pre-CEA groups, respectively (P < 0.01). We also examined the prognostic significance of other potential clinicopathologic factors (Table 2). The 5-year LRFS correlated with lymphovascular invasion, perineural invasion, TRG, ypT, ypN, and circumferential resection margin (CRM). The 5-year DMFS was associated with lower tumor border from the anal verge >7 cm, cT, cN, lymphovascular invasion, perineural invasion, ypT, ypN, CRM, and TRG. Similar results were obtained for DFS, with the exception of tumor location from the anal verge, which lacked prognostic significance.
FIGURE 2.
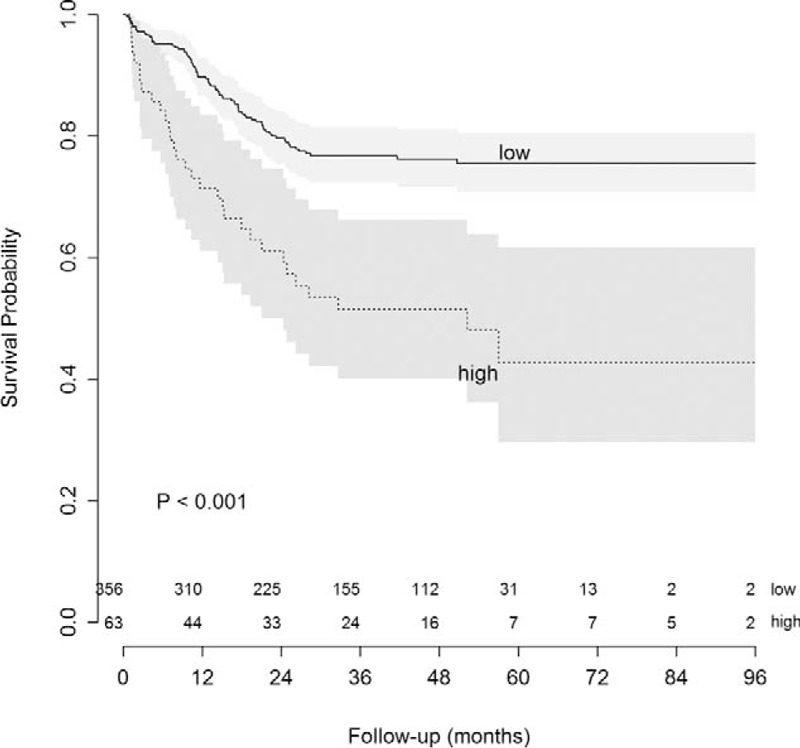
Distant metastasis-free survival according to pre-CEA level. pre-CEA=pretreatment serum carcinoembryonic antigen.
TABLE 2.
Univariate Analysis of Predictors on the Percentage of 5-Year Local Recurrence, Distant Metastasis, and Disease-Free Survival
Significant factors in univariate analysis were included in multivariate analysis (Table 3). Perineural invasion was the only prognostic factor associated with all outcomes (LRFS, DMFS, and DFS). In addition to perineural invasion, independent prognostic factors associated with DMFS included pre-CEA level, ypN, and ypT. In multivariate analysis, no other factor added significant prognostic information for the prediction of distant metastasis already considering perineural invasion, ypT, ypN, and pre-CEA level.
TABLE 3.
Multivariate Analysis of Different Covariables on 5-Year Local Recurrence Free-Survival, Distant Metastasis Free-Survival, and Disease Free-Survival After Preoperative CRT
DISCUSSION
The cumulative distant metastasis rate of rectal cancer with neoadjuvant CRT, followed by TME, has been reported as 25% to 40%.2,3,7,8 In our study, the major treatment failure was distant metastasis (26.0%) rather than local recurrence (9.3%), with isolated local recurrence (6.9%). These results question the current indiscriminate treatment strategy. Early systemic chemotherapy with newer biological agents is more likely to improve rates of distant metastasis and survival. Therefore, it is crucial to select high-risk patients who are more likely to experience systemic relapse and benefit from this treatment. In 1978, Wanebo et al14 reported an inverse linear relationship between serum CEA and estimated mean time to relapse in Duke B and C colorectal cancer. Since then, many studies have reported the prognostic value of pre-CEA on colorectal cancer outcomes.13,15–18,26,27
When analyzing the prognostic role of pre-CEA in rectal cancer treated with neoadjuvant CRT, however, there has been controversy concerning the significance of elevated CEA level associated with low DFS.15,28–32 The inconsistent results could be explained by different cutoff values of pre-CEA levels. All of these studies used arbitrarily defined cutoff values of 2.5 to 10 ng/mL. No value was based directly on oncologic outcome. In contrast, we evaluated the prognostic significance of determined cutoff values that could define a subgroup with the greatest survival difference by using a maximal χ2 method. In addition to the receiver operating characteristic, which assumes that all observations have occurred when the test is performed, maximally selected rank statistics allow for the evaluation of cutpoints with respect to a survival response. To our knowledge, this is the first study to use this method to determine the cutoff value of the pre-CEA level. Furthermore, most of the studies evaluated the correlation between pre-CEA and DFS and did not evaluate local or distant recurrence separately. In this study, pre-CEA level was only significantly associated with distant metastasis and not local recurrence, which is verified by previous studies.27,29 Park et al27 showed that although perioperative CEA was a significant prognostic indicator for systemic recurrence, it was unable to predict locoregional recurrence in either stage II or III patients. Similarly, Wang et al29 showed that the predictive value of pre-CEA on distant metastasis was more prominent in early systemic metastasis (within 6 months after surgery).
Another unique aspect to our study is the suggestion that current smoking history be considered in the evaluation of the clinical application of serum CEA. In the present study, the median pre-CEA level was significantly different according to smoking status. Using our calculated cutoff values of CEA for smokers and nonsmokers, the proportion of nonsmokers (14.2%) and smokers (17.9%) in the high-risk group was similar (P = 0.47). However, if the same cutoff value is used for smokers and nonsmokers (ie, using the 6.6 ng/mL value of the nonsmoking group), the proportion of high-risk patients in the smoking group would d increase to 34.3%, and is significantly different from nonsmoker group (P = 0.02).
We believe that prognostic effect of pre-CEA levels evaluated herein should be considered in future studies. First, according to the pre-CEA level, a more selective approach to neoadjuvant CRT should be considered for patients who are at a low risk of local recurrence, and systemic chemotherapy should be initiated as soon as possible.33,34 Williamson et al34 reported that the role of current neoadjuvant therapy could be diminished by performing surgery for highly selective patients. They selectively administered neoadjuvant CRT for rectal cancer with predicted CRM involvement by MRI precluding R0 resection and for extensive nodal disease (at least N2). Surprisingly, the 5-year local recurrence rate was 6.5% in the neoadjuvant CRT group and nil in the surgery-alone group (P = 0.04).34 Based on this result, it is thought that earlier systemic treatment is more likely to improve DMFS in this specific clinical setting. Second, although there remains no consensus about effective neoadjuvant and adjuvant chemotherapy regimens to reduce distant metastasis, the pre-CEA level could be used as an important stratification factor. A more detailed stratification of a patient cohort based on this study could be incorporated into a clinical study designed to test the efficacy of more intensive neoadjuvant and adjuvant chemotherapy. Finally, we believe that our results could provide important insights for the development of promising CEA-targeted treatment.35
There are some limitations to our study. The relatively small sample size and the relatively short follow-up (median 42.2 months) are potential limitations that might explain lack of results within certain subgroups. For example, we could not clearly discern the prognostic effect in patients with pCR. Patients with pCR and patients with low pre-CEA highly overlapped (45 of 49 patients with pCR were in the low CEA group), supporting previous reports that the pre-CEA level is a predictive factor for complete tumor response.31,36 Another limitation is that certain clinical factors, including tethered or fixed tumor, circumferential lesion, and near obstruction, were not evaluated in this study. Finally, although the physical examination remains an important factor in the treatment of locally advanced rectal cancer,32 significant interobserver variation and absence of physical examination records in some cases hindered a full consideration of this factor
In summary, the pre-CEA level is a unique factor that could predict distant metastasis before initiating any treatment, and moreover, could predict the response of neoadjuvant CRT in rectal cancer to some extent. Based on the observation made in this study, we recommend routine pre-CEA testing for all locally advanced rectal cancer patients. In addition, the cutoff value of pre-CEA should take into consideration the current smoking history of patients. The pre-CEA level appears to be a significant preoperative prognostic factor with a predictive role that is as valuable as any known pathologic factor. Future studies should consider pre-CEA when evaluating oncologic outcomes.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CRM = circumferential resection margin, CRT = chemoradiotherapy, CT = computed tomography, DFS = disease-free survival, DMFS = distant metastasis-free survival, LRFS = local recurrence-free survival, MRI = magnetic resonance image, pCR = pathologic complete response, TME = total mesorectal excision, TRG = tumor regression grade.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 3.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12:575–582. [DOI] [PubMed] [Google Scholar]
- 4.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341:457–460. [DOI] [PubMed] [Google Scholar]
- 5.Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000; 356:93–96. [DOI] [PubMed] [Google Scholar]
- 6.Rich T, Gunderson LL, Lew R, et al. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer 1983; 52:1317–1329. [DOI] [PubMed] [Google Scholar]
- 7.Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014; 15:1245–1253. [DOI] [PubMed] [Google Scholar]
- 8.Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clinl Oncol 2014; 32:1554–1562. [DOI] [PubMed] [Google Scholar]
- 9.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124:979–994. [DOI] [PubMed] [Google Scholar]
- 10.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001; 357:497–504. [DOI] [PubMed] [Google Scholar]
- 11.Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology 2004; 232:773–783. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clical Practice Guidelines in Oncology. Rectal Cancer, version 2.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf Accessed March 12, 2015. [Google Scholar]
- 13.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 1999; 9:67–81. [DOI] [PubMed] [Google Scholar]
- 14.Wanebo HJ, Rao B, Pinsky CM, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med 1978; 299:448–451. [DOI] [PubMed] [Google Scholar]
- 15.Kim CW, Yu CS, Yang SS, et al. Clinical significance of pre- to post-chemoradiotherapy s-CEA reduction ratio in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol 2011; 18:3271–3277. [DOI] [PubMed] [Google Scholar]
- 16.Wolmark N, Fisher B, Wieand HS, et al. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg 1984; 199:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison LE, Guillem JG, Paty P, et al. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg 1997; 185:55–59. [DOI] [PubMed] [Google Scholar]
- 18.Thirunavukarasu P, Sukumar S, Sathaiah M, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst 2011; 103:689–697. [DOI] [PubMed] [Google Scholar]
- 19.Gold P, Goldenberg N. The carcinoembryonic antigen (CEA): past, present, and future. McGill J Med 1998; 3:46–66. [Google Scholar]
- 20.Alexander JC, Silverman NA, Chretien PB. Effect of age and cigarette smoking on carcinoembryonic antigen levels. JAMA 1976; 235:1975–1979. [PubMed] [Google Scholar]
- 21.Fletcher RH. Carcinoembryonic antigen. Ann Intern Med 1986; 104:66–73. [DOI] [PubMed] [Google Scholar]
- 22.Kim CH, Kim HJ, Huh JW, et al. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J surg oncol 2014; 110:989–996. [DOI] [PubMed] [Google Scholar]
- 23.Huh JW, Lee JH, Kim HR. Pretreatment expression of 13 molecular markers as a predictor of tumor responses after neoadjuvant chemoradiation in rectal cancer. Ann Surg 2014; 259:508–515. [DOI] [PubMed] [Google Scholar]
- 24.Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23:8688–8696. [DOI] [PubMed] [Google Scholar]
- 25.Hothorn T. maxstat: Maximally Selected Rank Statistics. Rpackage version 0.7-12. Available at: http://CRAN.R-project.org/package=maxstat2007 Accessed March 12, 2015. [Google Scholar]
- 26.Nissan A, Stojadinovic A, Shia J, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated by surgery alone. J Clin Oncol 2006; 24:4078–4084. [DOI] [PubMed] [Google Scholar]
- 27.Park YA, Lee KY, Kim NK, et al. Prognostic effect of perioperative change of serum carcinoembryonic antigen level: a useful tool for detection of systemic recurrence in rectal cancer. Ann Surg Oncol 2006; 13:645–650. [DOI] [PubMed] [Google Scholar]
- 28.Moreno Garcia V, Cejas P, Blanco Codesido M, et al. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2009; 24:741–748. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Zhong XG, Peng YF, et al. Prognostic value of pretreatment level of carcinoembryonic antigen on tumour downstaging and early occurring metastasis in locally advanced rectal cancer following neoadjuvant radiotherapy (30 Gy in 10 fractions). Colorectal Dis 2014; 16:33–39. [DOI] [PubMed] [Google Scholar]
- 30.Perez RO, Sao Juliao GP, Habr-Gama A, et al. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum 2009; 52:1137–1143. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Lim SB, Kim DY, et al. Carcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgery. Int J Radiat Oncol Biol Phys 2009; 74:810–817. [DOI] [PubMed] [Google Scholar]
- 32.Myerson RJ, Singh A, Birnbaum EH, et al. Pretreatment clinical findings predict outcome for patients receiving preoperative radiation for rectal cancer. Int J Radiat Oncol Biol Phys 2001; 50:665–674. [DOI] [PubMed] [Google Scholar]
- 33.Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011; 253:711–719. [DOI] [PubMed] [Google Scholar]
- 34.Williamson JS, Jones HG, Davies M, et al. Outcomes in locally advanced rectal cancer with highly selective preoperative chemoradiotherapy. Br J Surg 2014; 101:1290–1298. [DOI] [PubMed] [Google Scholar]
- 35.Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann surg 2013; 258:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 2013; 56:698–703. [DOI] [PubMed] [Google Scholar]


