Abstract
The study aimed to identify predictors of severe acute hypertension (≥180/110 mmHg) during severe hypoglycemia and to assess the efficacy of prior use of catecholamine-blocking agents for preventing adverse influences in diabetic patients with severe hypoglycemia. We performed a retrospective study between January 2006 and March 2012 to assess diabetic patients with severe hypoglycemia at a single center in Japan. Severe hypoglycemia was defined as the presence of any hypoglycemic symptoms that required the medical assistance of another person after visiting the emergency room by ambulance. Multivariate logistic regression analysis was performed to identify possible predictors of severe hypertension due to severe hypoglycemia and to assess whether prior use of alpha- or beta-blockers is beneficial for the prevention of severe hypertension in diabetic patients with severe hypoglycemia. Multivariate adjustments were made for age, sex, preexisting hypertension, history of ischemic heart disease, blood glucose level upon arrival, estimated GFR, and prior use of alpha- or beta-blockers. A total of 59,602 patients who visited the emergency room were screened and 352 diabetic patients with severe hypoglycemia were enrolled. Incidences of severe hypertension before and at 3 and 6 hours after the initiation of antihypoglycemic treatment were 21.3%, 6.7%, and 0% in patients with type 1 diabetes (n = 61) and 38.8%, 18.2%, and 8.2% in patients with type 2 diabetes (n = 291), respectively. Aging was positively (odds ratio [OR], 1.02; 95% confidence interval [CI], 1.00−1.03; P = 0.02) and female sex was negatively (OR, 0.50; 95% CI, 0.29−0.86; P = 0.01) associated with occurrence of severe hypertension during severe hypoglycemia. In addition, prior use of beta-blockers was negatively associated with occurrence of severe hypertension during severe hypoglycemia using multivariate logistic regression analysis (OR, 0.31; 95% CI, 0.11−0.83; P = 0.02). None of the patients with prior use of beta-blockers had hypokalemia (<3.0 mEq/L). Prior use of beta-blockers may prevent adverse influences such as severe hypertension and hypokalemia during severe hypoglycemia in diabetic patients.
INTRODUCTION
Severe hypoglycemia is a life-threatening condition. Some reports have indicated that severe hypoglycemia is associated with a higher mortality in critically ill patients.1,2 In addition, several studies have suggested that severe hypoglycemia is associated with an increased risk of cardiovascular disease.3–5 One possible reason for this association is that severe hypoglycemia can lead to the activation of the sympathoadrenal system and release of counterregulatory hormones, such as norepinephrine and epinephrine, resulting in significant hemodynamic changes.6,7 Several studies have found markedly elevated systolic and diastolic blood pressure in patients with diabetes during severe hypoglycemia,5,8 which can lead to hypertensive emergencies, such as stroke, acute coronary syndrome, and aortic dissection.9–12 In addition, hypokalemia, which can lead to lethal arrhythmias, is often observed during severe hypoglycemia.5,13
Based on the pathophysiological mechanisms, prior use of catecholamine-blocking agents such as alpha- and beta-blockers may prevent adverse influence of hypersecretion of catecholamines induced by severe hypoglycemia. A recent study in rats reported that beta-adrenergic blockade significantly reduced cardiac arrhythmias and completely eliminated deaths due to severe hypoglycemia.13 However, no clinical study has investigated whether prior use of alpha- or beta-blockers (α/β-blockers) prevents acute elevation of blood pressure due to severe hypoglycemia. The aims of this study were to identify predictors of severe acute hypertension during severe hypoglycemia in diabetic patients and to assess the preventive efficacy of prior use of α/β-blockers.
METHODS
Study Design and Population
Patients with diabetes who were transported by ambulance and who presented with severe hypoglycemia at the National Center for Global Health and Medicine, Tokyo, Japan, between January 1, 2006 and March 31, 2012 were included in this retrospective study. Severe hypoglycemia was defined as the presence of any hypoglycemic symptoms that the patients were unable to resolve themselves and that required medical assistance during the emergency room visit.14 The measurement of blood glucose levels was mainly performed at a central laboratory (79%, 279/352); however, for some patients, these levels were measured using a blood glucose meter (21%, 73/352). Here, we evaluated the characteristics of patients, use of antihypertensive medications, and blood pressure before and after starting the antihypoglycemic treatment. All data, including clinical records and laboratory results, were independently reviewed by at least 2 diabetologists, and a 3rd diabetologist resolved all disagreements. A previous diagnosis of diabetes or the current use of antidiabetic medicines confirmed the presence of diabetes, which was further classified as type 1 diabetes (T1D), type 2 diabetes (T2D), or other types. Moreover, a previous diagnosis or the presence of antibodies to glutamic acid decarboxylase confirmed T1D. If the diagnosis was either previously made or specific causes were absent, then it was classified as T2D. A previous diagnosis or the current use of antihypertensive medications confirmed the condition of preexisting hypertension. The exclusion criterion of this study included patients with cardiopulmonary arrest upon arrival. The analyses of the data were performed using only the latest hospital visit for each individual. The institutional review board of the National Center for Global Health and Medicine Hospital granted approval for this study.
Blood Pressure and Other Measurements
Systolic and diastolic blood pressures were measured upon arrival and were rechecked 3 and 6 hours after beginning the antihypoglycemic treatment, given that antihypertensive or vasopressor drugs were not used during this period. Severe hypertension was considered when the systolic blood pressure was ≥180 mmHg and/or diastolic blood pressure was ≥110 mmHg.12 In patients with preexisting hypertension, the daily use of antihypertensive drugs was checked. Antihypertensive drugs were classified into the following categories: angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, alpha-blockers, beta-blockers, or diuretics. Serum creatinine and potassium levels were measured upon arrival. Furthermore, the glycated hemoglobin (HbA1c) level was measured at the nearest time within 3 months of the arrival. As recommended by the Japanese Society of Nephrology, the following formula was used to calculate the estimated glomerular filtration rate (eGFR): eGFR (mL/min/1.73 m2) = 194 × Cre−1.094 × Age−0.287 (×0.739 if the patient was a female).15 A serum potassium level of <3.0 mEq/L was considered to be a hypokalemic indicator.
Statistical Methods
Data are presented as the number, percentage, or median with lower and upper limits of the interquartile range. Continuous variables were compared using Wilcoxon rank sum tests. Categorical variables were compared using Chi-square tests or Fisher exact tests. Patients were divided into 2 groups according to the presence or absence of preexisting hypertension (≥180/110 mmHg). In addition, patients with preexisting hypertension were divided according to the use or nonuse of alpha- or beta-blockers. Multivariate logistic regression analysis was performed to identify possible predictors of severe hypertension due to severe hypoglycemia and to assess whether prior use of alpha- or beta-blockers is beneficial for the prevention of severe hypertension in diabetic patients with severe hypoglycemia. Multivariate adjustments were made for age, sex, preexisting hypertension, history of ischemic heart disease, blood glucose level upon arrival, eGFR, and prior use of alpha- or beta-blockers. P values <0.05 according to 2-sided tests were considered statistically significant. Variables derived from 5 or fewer patients were excluded from comparative analyses. All the analyses were performed using Stata software, version 11.1 (Stata Corp, College Station, TX).
RESULTS
A total of 59,602 cases that visited the emergency room by ambulance between January 1, 2006 and March 31, 2012 were screened, and 352 diabetic patients with severe hypoglycemia met the criteria for study inclusion. Median systolic and diastolic blood pressures during severe hypoglycemia were 164 (141 − 190) mmHg and 78 (65 − 96) mmHg, respectively; 35.8% of these patients had severe hypertension. The clinical characteristics of T1D and T2D patients upon arrival are presented in Table 1. In the T1D patients (n = 61), 21.3% had severe hypertension during severe hypoglycemia. Age was nonsignificantly older in the T1D patients with severe hypertension than those without it. Neither blood glucose level upon arrival nor HbA1c level differed significantly between T1D patients with and without severe hypertension. However, prevalence of preexisting hypertension was more than 2 times higher and eGFR significantly lower in the T1D patients with severe hypertension than those without it. Although alpha-blocker use was not observed in the T1D patients, beta-blocker use was observed but only in the T1D patients without severe hypertension. In the T2D patients (n = 291), 38.8% had severe hypertension during severe hypoglycemia. Blood glucose, HbA1c level, and duration of diabetes did not differ significantly between T2D patients with and without severe hypertension. Both alpha- and beta-blockers were used more frequently by patients without severe hypertension compared to those with it but the difference was not significant. All study patients were treated by glucose infusion.
TABLE 1.
Characteristics of Type 1 and Type 2 Diabetes Patients With Severe Hypoglycemia Upon Arrival∗
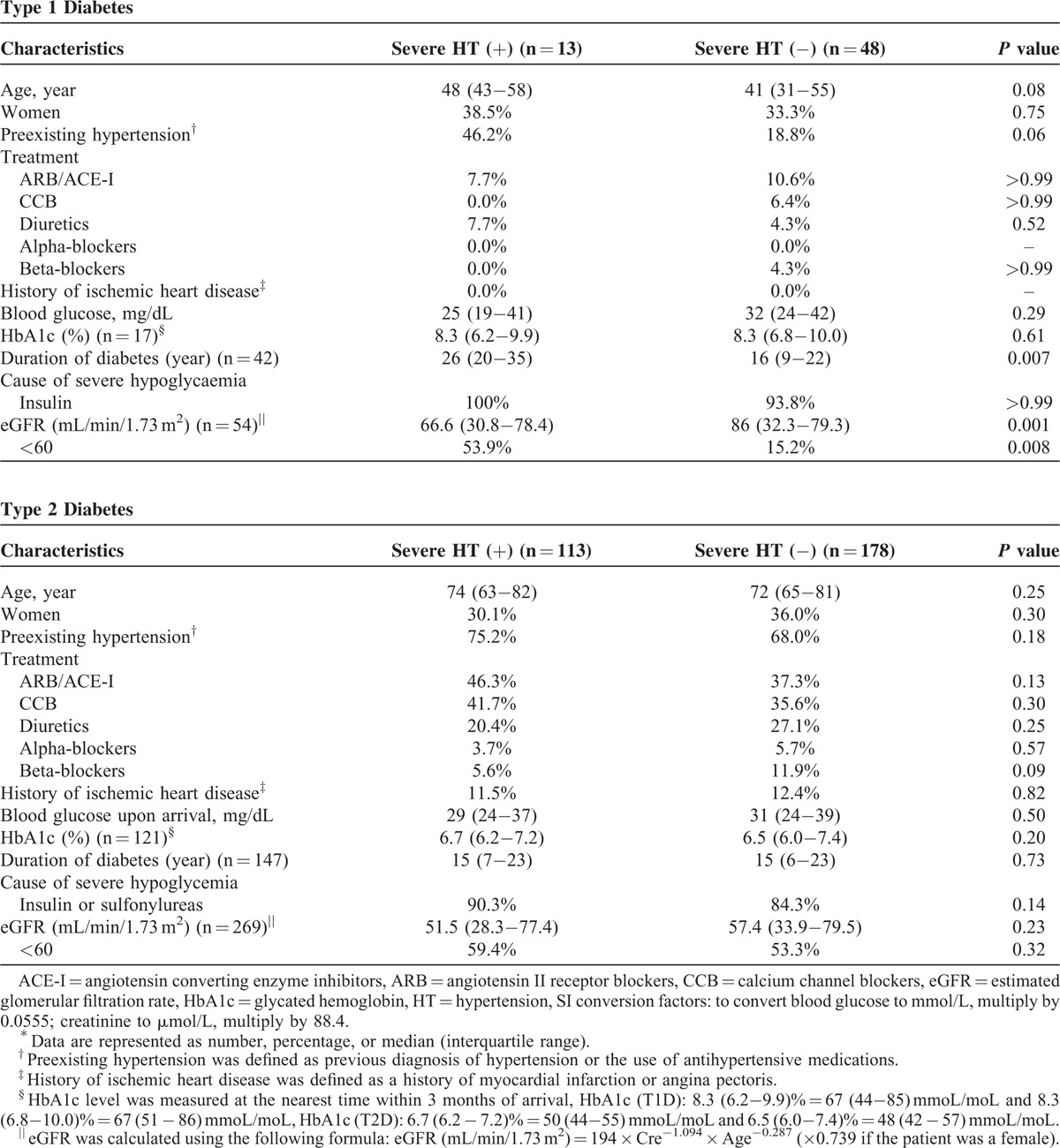
Incidences of severe hypertension before and after the initiation of anti-hypoglycemic treatment are shown in Figure 1 for both T1D and T2D patients with severe hypoglycemia. Severe hypertension during severe hypoglycemia was more complicated in the T2D patients than in the T1D patients (Figure 1A). Severe hypertension was still observed in 8.2% of the T2D patients but in none of the T1D patients at 6 hours after onset of treatment. Among patients with preexisting hypertension, incidences of severe hypertension did not differ significantly between T1D and T2D patients (Figure 1B). However, among patients without preexisting hypertension, incidences of severe hypertension before and after treatment onset were higher in the T2D patients than in the T1D patients (Figure 1C).
FIGURE 1.
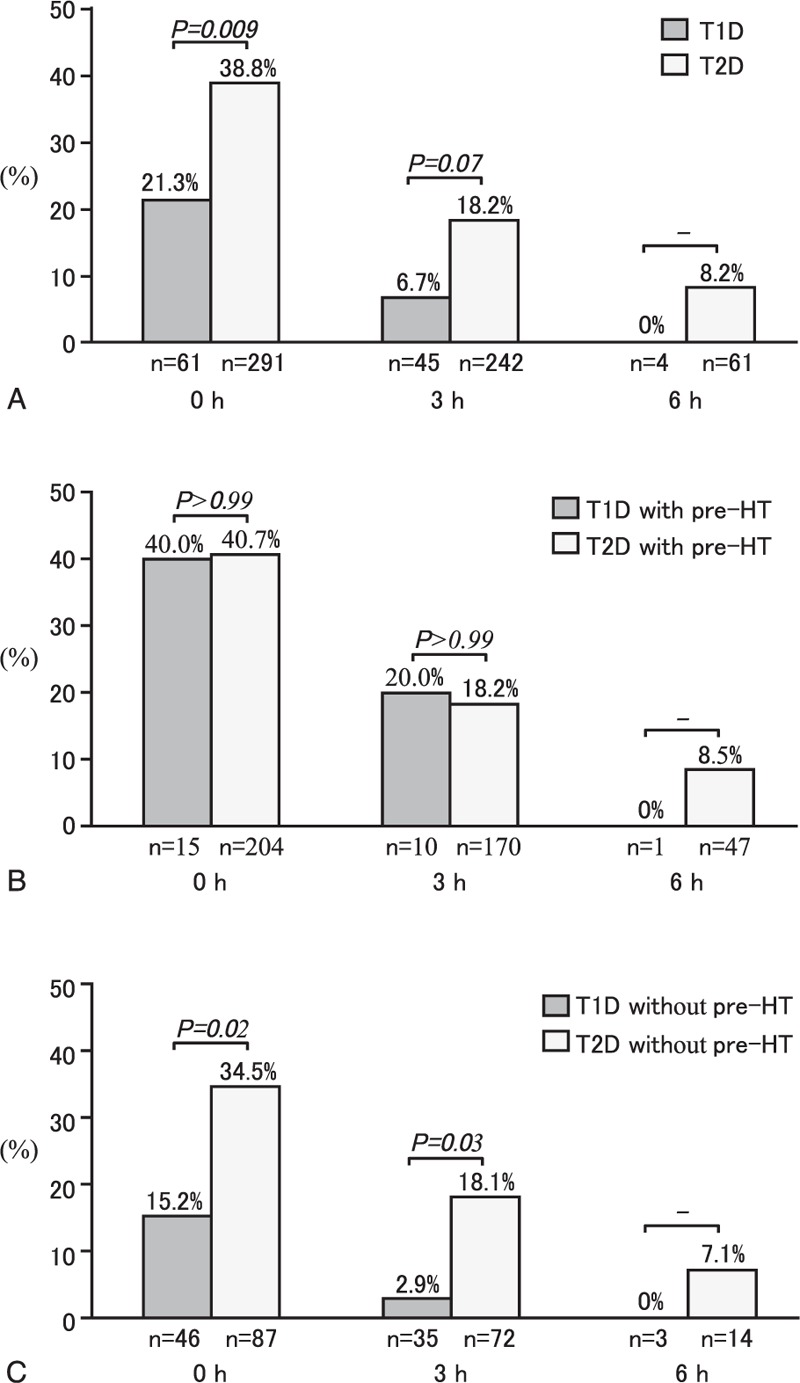
Incidence of severe hypertension before and after the initiation of treatment for severe hypoglycemia. Incidences of severe hypertension in all T1D and T2D patients (Panel A). Incidences of severe hypertension in T1D and T2D patients with preexisting hypertension (Panel B). Incidences of severe hypertension in T1D and T2D patients without preexisting hypertension (Panel C). T1D = type 1 diabetes, T2D = type 2 diabetes, pre-HT = preexisting hypertension.
Incidences of severe hypertension in diabetic patients with or without prior use of α/β-blockers are presented in Figure 2A. Among the T1D patients, incidence of severe hypertension was significantly higher in patients with preexisting hypertension who did not use α/β-blockers than those without preexisting hypertension (50.0% vs 15.2%; P = 0.01). On the other hand, none of the T1D patients with preexisting hypertension who used α/β-blockers had severe hypertension during severe hypoglycemia. Similarly, incidence of severe hypertension was higher in the T2D patients with preexisting hypertension who did not use α/β-blockers than in T2D patients without preexisting hypertension (42.9% vs 34.5%; P = 0.12). Among the T2D patients with preexisting hypertension, incidence of severe hypertension was significantly lower in patients with prior use of α/β-blockers than in nonusers (24.3% vs 42.9%; P = 0.03). Moreover, in both T1D and T2D patients, incidence of severe hypertension was lower in patients with prior use of α/β-blockers than in patients without preexisting hypertension. Figure 2B presents incidence of severe hypertension before and after the treatment initiation for all diabetes patients with preexisting hypertension who had or had not used α/β-blockers. Incidence of severe hypertension during severe hypoglycemia was significantly lower in patients with prior use of α/β-blockers than in those without it (23.1% vs 43.4%; P = 0.01), whereas incidence of severe hypertension at 3 and 6 hours after the treatment initiation did not differ significantly between patients with and without prior use of α/β-blockers. Although incidences of severe hypertension before and after the treatment did not differ significantly between patients with and without prior use of alpha-blockers (Figure 2C), incidences of severe hypertension in those with beta-blockers were similar to those of α/β-blocker users (Figure 2D).
FIGURE 2.
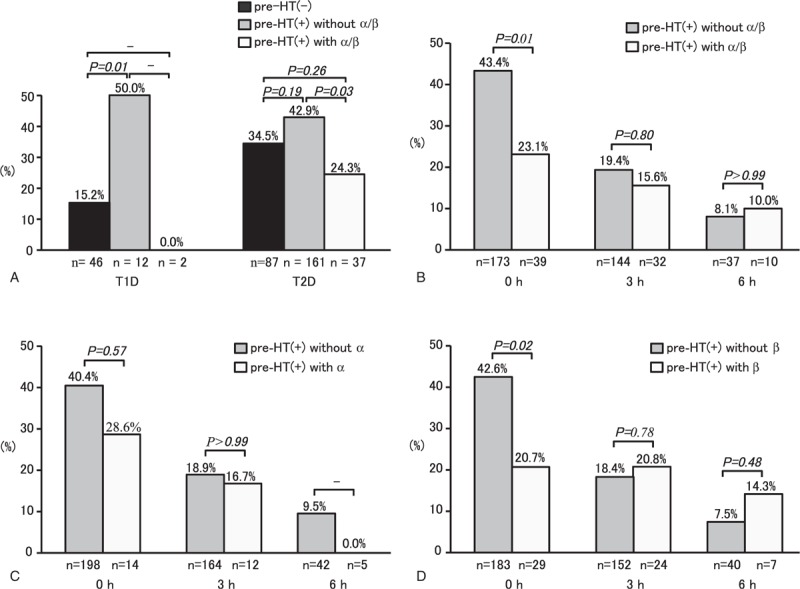
Incidence of severe hypertension before and after initiation of treatment for severe hypoglycemia in diabetic patients with or without prior use of alpha- and beta-blockers. Incidences of severe hypertension during severe hypoglycemia in T1D and T2D patients (Panel A). Incidences of severe hypertension before and after initiation of treatment for severe hypoglycemia in patients with or without prior use of α/β-blockers (Panel B). Incidences of severe hypertension after initiation of treatment for severe hypoglycemia in patients with or without prior use of α-blockers (Panel C). Incidences of severe hypertension after initiation of treatment for severe hypoglycemia in patients with or without prior use of β-blockers (Panel D). T1D = type 1 diabetes, T2D = type 2 diabetes, pre-HT = preexisting hypertension, α/β = alpha- or beta-blockers, α = alpha-blockers, β = beta-blockers.
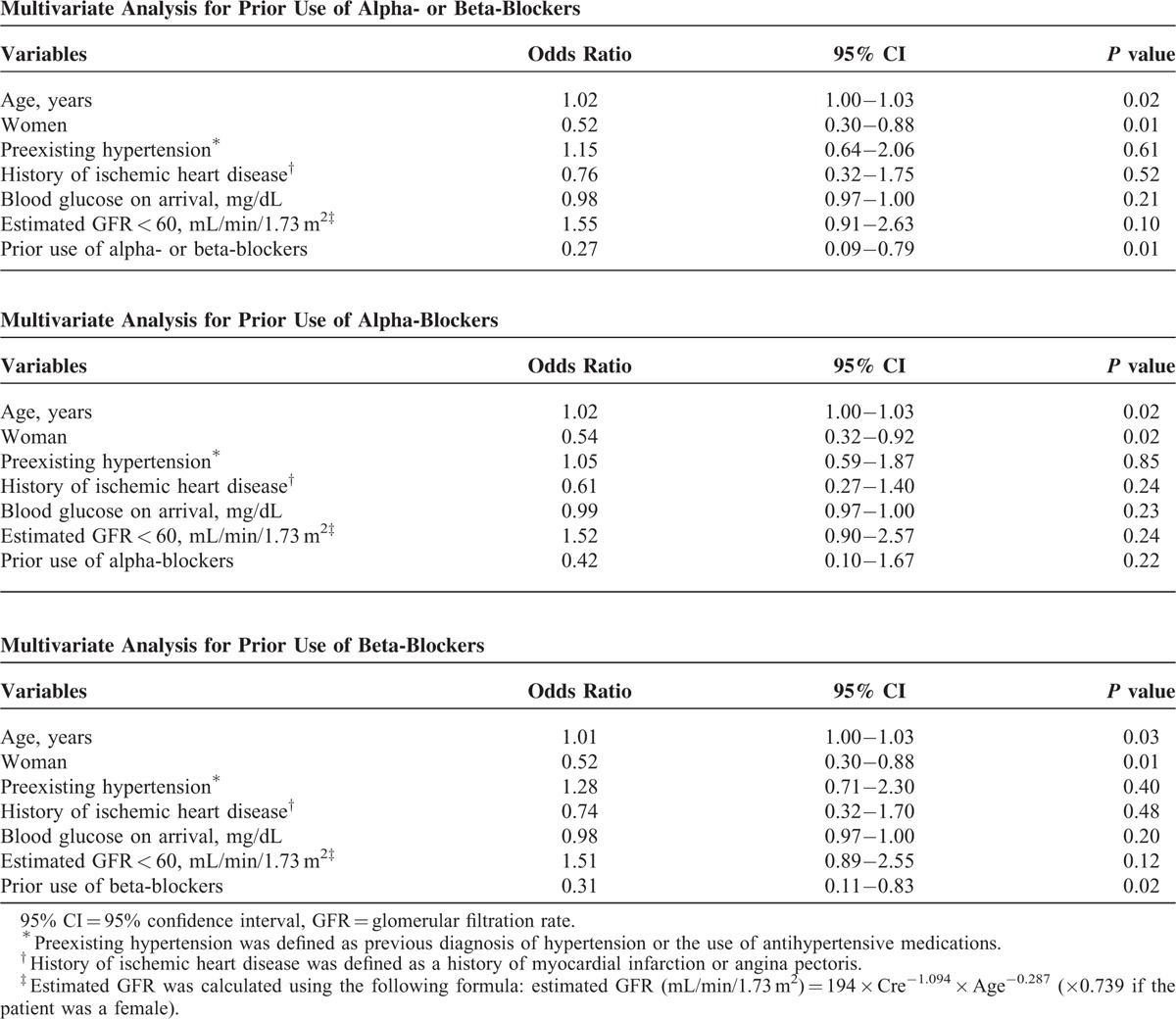
Multivariate analyses for incidence of severe hypertension during severe hypoglycemia in all diabetic patients with preexisting hypertension are presented in Table 2. After multivariate adjustment, prior use of α/β-blockers was significantly and negatively associated with occurrence of severe hypertension (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.11–0.68; P = 0.005). In addition, age was positively (OR, 1.02; 95% CI, 1.00–1.03; P = 0.02) and female sex was negatively (OR, 0.50; 95% CI, 0.29–0.86; P = 0.01) associated with occurrence of severe hypertension. Although there was no significant association between prior use of alpha-blockers and occurrence of severe hypertension during severe hypoglycemia, there was significant negative association between prior use of beta-blockers and occurrence of severe hypertension (OR, 0.31; 95% CI, 0.11–0.79; P = 0.01). Significant negative associations between occurrence of severe hypertension and prior use of α/β- and of beta-blockers were also observed in analysis limited to T2D patients.
TABLE 2.
Multivariate Analysis for Severe Hypertension During Severe Hypoglycemia in Diabetic Patients With Preexisting Hypertension
Additional analyses were conducted to access the efficacy of beta-blockers for preventing other adverse events during severe hypoglycemia, such as hypokalemia.5,13 There was a strong trend for reduced incidence of hypokalemia in patients with prior use of beta-blockers compared to those without it (0.0% vs 11.6%; P = 0.06).
DISCUSSION
Severe hypertension was often observed both before and after the initiation of glucose infusion treatment for severe hypoglycemia. In addition, aging and male sex were positively associated with incidence of severe hypertension during severe hypoglycemia. However, both T1D and T2D patients who had used α/β-blockers, particularly beta-blockers, prior to the event of severe hypoglycemia demonstrated significantly lower incidence of severe hypertension. Moreover, none of the diabetic patients with prior use of beta-blockers had hypokalemia during severe hypoglycemia. To our knowledge, this is the first study to report that prior use of beta-blockers may be effective for the prevention of dangerously elevated blood pressure and hypokalemia during severe hypoglycemia in diabetic patients in a clinical setting.
The present study suggests that severe hypertension can last for several hours, even after initiation of antihypoglycemic treatment. Considering the time from occurrence of severe hypoglycemia to the initiation of treatment, severe hypertension may have continued for much longer in some diabetic patients. Incidence of severe hypertension associated with severe hypoglycemia was significantly lower in T1D patients than T2D patients. In addition to distinct pathophysiologies and background complications, this difference may be partly attributed to the fact that T1D patients typically experience more frequent hypoglycemic episodes, which blunts the counterregulatory response due to hypoglycemia-associated autonomic failure.16,17 Incidences of severe hypertension at 3 and 6 hours after the initiation of treatment did not differ significantly between diabetic patients with and without prior use of beta-blockers, possibly because the late phase of hypoglycemia-associated hypertension is more attributable to hypersecretions of cortisol and growth hormone than to excessive catecholamine release.
Severe hypoglycemia-induced sympathoadrenal activation can lead to serious cardiovascular complications,5,13 so prior use of beta-blockers may have the potential effect on prevention of lethal cardiovascular events. Indeed, a recent study in rats indicated that prior use of beta-blockers markedly reduced cardiac arrhythmias and mortality associated with severe hypoglycemia.13 Systolic and diastolic blood pressures during severe hypoglycemia were also significantly lower in beta-blocker infused rats compared to controls.13 These results are consistent with our current findings and support the study outcome. Furthermore, hypokalemia has also been observed during severe hypoglycemia,5,13 possibly because not only hyperinsulinemia but also secretion of catecholamines drives potassium into the cell during hypoglycemia. Prevention of hypokalemia during severe hypoglycemia by prior use of beta-blockers may thus decrease the risk of lethal arrhythmias.
Alternatively, use of beta-blockers by patients with diabetes could theoretically increase the risk of hypoglycemia and hypoglycemia unawareness. However, there is little evidence to support the assertion that beta-blockers should be routinely contraindicated in diabetes as they have few clinically important effects on hypoglycemia unawareness and recovery.18–25 Although some hypoglycemia symptoms like tremor and palpitation may be blunted,18 symptoms such as sweating may be enhanced.18–20 The reason is that sweating is a sympathetic cholinergic response to hypoglycemia, which cannot be suppressed by β-blockers, and prior use of β-blockers enhances sympathoadrenal activation by hypoglycemia.13,26 In addition, total symptoms of hypoglycemia may not be influenced substantially by β-blockers.20–22 Furthermore, some studies suggest that β-blockers, particularly β1-selective β-blockers, have little impact on the risk of hypoglycemia and its recovery.22–25,27 Therefore, selective β-blockers should be preferentially used for diabetic patients with cardiovascular diseases not only to improve long-term cardiovascular outcome, but also for potentially life-saving effects during severe hypoglycemia.28–30 Further studies are needed to assess the advantages and disadvantages of β-blockers for patients with diabetes.
Aging was positively and female sex was negatively associated with occurrence of severe hypertension during severe hypoglycemia. Both increased sympathetic nervous system activity with age31,32 and reduced neuronal reuptake or systemic plasma clearance of norepinephrine in older patients may result in higher norepinephrine concentrations in response to severe hypoglycemic stress.33–35 See comment in PubMed Commons below Studies of perimenopausal women have suggested that estrogen administration attenuates total body norepinephrine spillover and vascular responses to intraarterial norepinephrine.36,37 Moreover, estrogen supplementation attenuated stress-induced increases in systolic and diastolic blood pressure, norepinephrine, and epinephrine.38 Thus, estrogen may protect against severe hypoglycemia, thereby explaining the influence of sex on hypoglycemia-induced hypertension risk.
Our study has several limitations. First, this is an observational study performed at a single national center. Second, missing data and limited samples may have influenced the results and the statistical analyses. In addition, patients with prehospital cardiopulmonary arrest could not be examined. Some patients with severe hypoglycemia may have died in prehospital settings, so further study is required to investigate the potential benefits of prior use of β-blockers for preventing lethal arrhythmias and dead-in-bed syndrome.39
In conclusion, diabetic patients who had used beta-blockers prior to the event of severe hypoglycemia showed lower incidence of severe hypertension and hypokalemia. We suggest that incorporation of β-blockers for the management of diabetes may reduce the potential dangers associated with severe hypoglycemia.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 26860701.
Footnotes
Abbreviations: CI = confidence interval, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, OR = odds ratio, T1D = type 1 diabetes, T2D = type 2 diabetes.
Ethics approval was obtained from the institutional review board of the National Center for Global Health and Medicine in Tokyo, Japan.
TT conceived the study and designed the protocol. TT, RY-H, HK, MKi, RH, and AK contributed to the data collection and manuscript preparation. TT, RY-H, MKi, HN, and MN analyzed all the data. TT, RY-H, MKa, and MN wrote the report. All authors contributed to the interpretation of results and approved the final version. MN had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by JSPS KAKENHI Grant Number 26860701.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007; 35:2262–2267. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Prediction of 90-day mortality in patients without diabetes by severe hypoglycemia: blood glucose level as a novel marker of severity of underlying disease. Acta Diabetol 2015; 52:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418. [DOI] [PubMed] [Google Scholar]
- 4.Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347:f4533. [DOI] [PubMed] [Google Scholar]
- 5.Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014; 37:217–225. [DOI] [PubMed] [Google Scholar]
- 6.Hilsted J, Bonde-Petersen F, Norgaard MB, et al. Haemodynamic changes in insulin-induced hypoglycaemia in normal man. Diabetologia 1984; 26:328–332. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BM, Gillen G, Dargie HJ, et al. The effects of insulin-induced hypoglycaemia on cardiovascular function in normal man: studies using radionuclide ventriculography. Diabetologia 1987; 30:841–845. [DOI] [PubMed] [Google Scholar]
- 8.Feldman-Billard S, Massin P, Meas T, et al. Hypoglycemia-induced blood pressure elevation in patients with diabetes. Arch Intern Med 2010; 170:829–831. [DOI] [PubMed] [Google Scholar]
- 9.Mayer SA, Kurtz P, Wyman A, et al. Clinical practices, complications,;1; and mortality in neurological patients with acute severe hypertension: the Studying the Treatment of Acute hyperTension registry. Crit Care Med 2011; 39:2330–2336. [DOI] [PubMed] [Google Scholar]
- 10.Lau J, Antman EM, Jimenez-Silva J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 1992; 327:248–254. [DOI] [PubMed] [Google Scholar]
- 11.Pretre R, Von Segesser LK. Aortic dissection. Lancet 1997; 349:1461–1464. [DOI] [PubMed] [Google Scholar]
- 12.Szczech LA, Granger CB, Dasta JF, et al. Acute kidney injury and cardiovascular outcomes in acute severe hypertension. Circulation 2010; 121:2183–2191. [DOI] [PubMed] [Google Scholar]
- 13.Reno CM, Daphna-Iken D, Chen YS, et al. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013; 62:3570–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes A. Standards of medical care in diabetes-2012. Diabetes Care 2012; 35 Suppl 1:S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 16.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002; 45:937–948. [DOI] [PubMed] [Google Scholar]
- 17.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004; 350:2272–2279. [DOI] [PubMed] [Google Scholar]
- 18.Kerr D, MacDonald IA, Heller SR, et al. Beta-adrenoceptor blockade and hypoglycaemia. A randomised, double-blind, placebo controlled comparison of metoprolol CR, atenolol and propranolol LA in normal subjects. Br J Clin Pharmacol 1990; 29:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molnar GW, Read RC. Propranolol enhancement of hypoglycemic sweating. Clin Pharmacol Ther 1974; 15:490–496. [DOI] [PubMed] [Google Scholar]
- 20.Viberti GC, Keen H, Bloom SR. Beta blockade and diabetes mellitus: effect of oxprenolol and metoprolol on the metabolic, cardiovascular, and hormonal response to insulin-induced hypoglycemia in normal subjects. Metabolism 1980; 29:866–872. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch IB, Boyle PJ, Craft S, et al. Higher glycemic thresholds for symptoms during beta-adrenergic blockade in IDDM. Diabetes 1991; 40:1177–1186. [DOI] [PubMed] [Google Scholar]
- 22.Barnett AH, Leslie D, Watkins PJ. Can insulin-treated diabetics be given beta-adrenergic blocking drugs? Br Med J 1980; 280:976–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shorr RI, Ray WA, Daugherty JR, et al. Antihypertensives and the risk of serious hypoglycemia in older persons using insulin or sulfonylureas. JAMA 1997; 278:40–43. [PubMed] [Google Scholar]
- 24.Thamer M, Ray NF, Taylor T. Association between antihypertensive drug use and hypoglycemia: a case-control study of diabetic users of insulin or sulfonylureas. Clin Ther 1999; 21:1387–1400. [DOI] [PubMed] [Google Scholar]
- 25.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ 1998; 317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 26.Corrall RJ, Frier BM, Davidson NM, et al. Cholinergic manifestations of the acute autonomic reaction to hypoglycaemia in man. Clin Sci (Lond) 1983; 64:49–53. [DOI] [PubMed] [Google Scholar]
- 27.Lager I, Blohme G, Smith U. Effect of cardioselective and non-selective beta-blockade on the hypoglycaemic response in insulin-dependent diabetics. Lancet 1979; 1:458–462. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Marciniak TA, Radford MJ, et al. Beta-blocker therapy for secondary prevention of myocardial infarction in elderly diabetic patients. Results from the National Cooperative Cardiovascular Project. J Am Coll Cardiol 1999; 34:1388–1394. [DOI] [PubMed] [Google Scholar]
- 29.Kjekshus J, Gilpin E, Cali G, et al. Diabetic patients and beta-blockers after acute myocardial infarction. Eur Heart J 1990; 11:43–50. [DOI] [PubMed] [Google Scholar]
- 30.Jonas M, Reicher-Reiss H, Boyko V, et al. Usefulness of beta-blocker therapy in patients with non-insulin-dependent diabetes mellitus and coronary artery disease. Bezafibrate Infarction Prevention (BIP) Study Group. Am J Cardiol 1996; 77:1273–1277. [DOI] [PubMed] [Google Scholar]
- 31.Ebert TJ, Morgan BJ, Barney JA, et al. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol 1992; 263:H798–H803. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein DS, Lake CR, Chernow B, et al. Age-dependence of hypertensive-normotensive differences in plasma norepinephrine. Hypertension 1983; 5:100–104. [DOI] [PubMed] [Google Scholar]
- 33.Esler M, Kaye D, Thompson J, et al. Effects of aging on epinephrine secretion and regional release of epinephrine from the human heart. J Clin Endocrinol Metab 1995; 80:435–442. [DOI] [PubMed] [Google Scholar]
- 34.Veith RC, Featherstone JA, Linares OA, et al. Age differences in plasma norepinephrine kinetics in humans. J Gerontol 1986; 41:319–324. [DOI] [PubMed] [Google Scholar]
- 35.Morrow LA, Linares OA, Hill TJ, et al. Age differences in the plasma clearance mechanisms for epinephrine and norepinephrine in humans. J Clin Endocrinol Metab 1987; 65:508–511. [DOI] [PubMed] [Google Scholar]
- 36.Sudhir K, Elser MD, Jennings GL, et al. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension 1997; 30:1538–1543. [DOI] [PubMed] [Google Scholar]
- 37.Sudhir K, Jennings GL, Funder JW, et al. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 1996; 28:330–334. [DOI] [PubMed] [Google Scholar]
- 38.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab 1999; 84:606–610. [DOI] [PubMed] [Google Scholar]
- 39.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med 1991; 8:49–58. [DOI] [PubMed] [Google Scholar]


