Abstract
Purpose: Diabetes mellitus as a main risk-factor of ischemic heart disease may interfere with postconditioning’scardioprotective effects. This study aimed to investigate the involvement of glycogen synthase kinase-3β (GSK-3β) and oxidation status in chronic diabetes-induced loss of cardioprotective effect of ischemic-postconditioning (IPostC) in Wistar rats.
Methods: After 8 weeks of induction of diabetes by streptozotocin (50mg/kg), hearts of control and diabetic rats were isolated and mounted on a constant-pressure Langendorff system. All hearts were subjected to 30min regional ischemia followed by 60min reperfusion (by occluding and re-opening of left anterior descending coronary artery, respectively). IPostC was applied immediately at the onset of reperfusion. At the end of reperfusion, the infarct size of myocardium was measured via computerized planimetry. Myocardial contents of malondealdehyde and glutathione as indices of oxidative status were assayed spectrophotometrically and the total and phosphorylated forms of myocardial GSK-3β were quantified through western blotting.
Results: IPostC reduced the infarct size of control hearts from 41±2.9% to 28±1.9% (P<0.05), whereas it could not induce significant changes in infarct size of diabetic animals (35±1.8% vs. 39±3.1%). IPostC-induced reduction in malondealdehyde and elevation in glutathione contents were significant only in control not in diabetic hearts. The total forms of GSK-3β were similar in all groups; however, the phosphorylation of GSK-3β (at Ser9) by IPostC was greater in control hearts than diabetics (P<0.01).
Conclusion: The failure of cardioprotection by IPostC in diabetic hearts may be attributed to the loss of phosphorylation of GSK-3β and thereby increase in oxidative stress in diabetic states.
Keywords: Reperfusion injury, Postconditioning, GSK-3β, Diabetes, Cardioprotection
Introduction
Ischemic heart disease (IHD) is the main cause of mortality in human societies and cardioprotection against ischemia is a matter of life and death in clinics.1,2 Ischemic postconditioning (IPostC) is one of the cardioprotective strategies introduced in recent years which had significant effect on reduction of infarct size and improvement of cardiac function after ischemia-reperfusion (IR) injury.3-5 In IPostC strategy, short episodes of reperfusion and ischemia are applied to the heart exactly after a long term ischemia.6 According to previous studies, the protective effect of this therapeutic strategy is decreased in pathologic conditions like diabetes mellitus, metabolic syndrome, arthrosclerosis, and hypertension.7,8
Diabetes mellitus is one of the main risk factors of cardiovascular diseases and there are evidences that diabetes intensify the myocardial ischemic damages. Considering the progressive increase of diabetes prevalence in different societies and the high rate of mortality among diabetic patients due to myocardial infarction, cardioprotection against IR injuries in diabetic patients is very important.9,10
IPostC shows its protective effects through different mechanisms, including activation of certain ion channels and receptors, phosphorylation of intracellular protein kinases, opening/closing of mitochondrial pores, and inhibiting cell necrosis and apoptosis.4,11 Pathologic conditions like diabetes, especially in chronic phases, may interfere with IPostC in cardioprotection, by changing any one of these mechanisms.
Glycogen synthase kinase-3β (GSK-3β) is a protein kinase plays an important role in most pathologic cellular processes like apoptosis, oxidative stress and mitochondrial dysfunction and is active in several disease conditions like diabetes, Alzheimer, ischemia, so forth.12-14 GSK-3β, in the case of activation, may open mitochondrial permeability transition pores (mPTP), leading to the activation of apoptotic factors and oxidative stress mediators.15 Oxidative stress is another main cause in the pathophysiology of IR injuries.16 There is a direct link between the activity of GSK-3β, opening of mPTP and production of reactive oxygen species and oxidative stress in the cell.17 According to direct involvement of GSK-3β and oxidative stress in the pathology of diabetes and myocardial ischemia, it is probable that these same factors are of the main mechanisms in interference of chronic diabetes with protective effect of IPostC in ischemic heart. As a result, the present study aimed to investigate the involvement of GSK-3β as well as glutathione and lipid peroxidation in the failure of IPostC on cardioprotection of diabetic hearts against IR injury.
Materials and Methods
Animals
Male Wistar rats (250–300 g body weight) were used in this study and had free access to food and water at all times before the start of the experiments. They were housed in laboratory room with a 12-h dark and 12-h light cycle. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85-23, revised 1996) and was approved by the local Animal Care Committee.
Induction of Diabetes
Diabetes was induced by a single injection of streptozotocin (50 mg/kgbody weight, i.p.) in diabetic groups. Development of the diabetes was confirmed 72h later by measuring blood glucose levels using a glucometer device through the sampling of blood with small scratching of tail of rats. The animals with blood glucose levels higher than 300 mg/dl were considered as diabetic. After 8 weeks of the disease, the diabetic animals as well as the controls were sacrificed and all experiments were performed in isolated perfused hearts.18
Surgical Preparation and Isolated Heart Perfusion
Surgical preparation was performed as described previously.18 All animals were heparinized (500 IU) and anesthetized with a mixture of ketamine (60 mg/kg) and xylosine (10 mg/kg). The hearts were rapidly excised, then mounted on the Langendorff perfusion apparatus and retrogradely perfused via the aorta with a Krebs–Henseleit solution (in mM/l: NaCl 118; KCl 4.7; CaCl2 2.5; MgSO4. 1.2; NaHCO3 25; KH2PO4 1.2; glucose 11.1) at a constant perfusion pressure of 75 mmHg and pH 7.4. The perfusion solution was gassed with a mixture of 95% O2, 5% CO2 at 37°C.
Induction of Regional Ischemia and Reperfusion
At the onset of experiment, a 5-0 silk thread was placed around the left anterior descending (LAD) coronary artery, close to its origin. After stabilization period of 15 min, all hearts were subjected to regional ischemia for 30 min followed by reperfusion for 60 min. Regional ischemia and reperfusion were induced by occluding and re-opening of LAD, respectively. An immediate fall in coronary flow at the onset of index ischemia (to about 30-40% of its baseline value) and its recovery upon reperfusion served as evidences of effective coronary occlusion and reperfusion.18 Ischemic postconditioning (IPostC) strategy in this study was three cycles of 30s reperfusion and ischemia (3 cycles of 30s R/I), respectively, immediately at the onset of reperfusion.
Experimental Protocol
The animals were divided into six groups (n=7/each) as following (the experiment was done in two series; one for infarct size study and another for biochemical assays):
1) Control (C); in which after surgical preparation and stabilization period, the isolated hearts of non-diabetic animals were subjected to 30 min ischemia and 60 min reperfusion.
2) Control with ischemic postconditioning (C+IPostC); in which the condition was similar to control group except that at the onset of reperfusion, the hearts were received 3 cycles of 30s R/I.
3) Diabetic (D); in which after surgical preparation and stabilization period, the isolated hearts of 8-week diabetic animals were subjected to 30 min ischemia and 45 min reperfusion.
4) Diabetic with ischemic postconditioning (D+IPostC); in which the condition was similar to diabetic group except that at the onset of reperfusion, the hearts were received 3 cycles of 30s R/I.
Infarct Size Measurement
After the end of reperfusion period, the coronary ligature was retightened at the same site and 0.25% Evance blue dye was infused via the side arm of aortic cannula into the coronary system to identify the risk zone as unstained from intact part of myocardium. The hearts were then frozen at -20°C and thereafter cut into thin slices (1 mm) from the apex to the base. The slices were incubated in 1% triphenyltetrazolium chloride (TTC) in phosphate buffer solution, pH 7.4 for 10 min at 37°C. The heart slices were immersed in 10% formalin for 24h to identify viable myocardium as red stained while necrotic tissue remains pale gray. The risk zone and the infarct area were determined by the computerized planimetry (Summa Sketch III, LandCalc). Infarct and risk area volumes were expressed in cubic millimeters and the percent of infarct to risk zone ratio was calculated.18
Preparation of Tissue Homogenates for Western Blot and Biochemical Analysis
At the end of the second series of experiment, the hearts from the ischemic part of left ventricle were sampled and immediately frozen in liquid nitrogen and stored at -80°C. The ventricular tissue was weighted and cut into pieces in ice-cold lysis buffer containing (in mmol) 1 KH2PO4, 1 KCL, 50 Tris-HCl pH 7.4, 1 EDTA, 1 NaF, 1 Na3VO4, and triton X100 and protease inhibitor cocktail and then homogenized with a Polytron PT-10/ST homogenizer. The homogenate was centrifuged at 10000 g for 10 min at 4°C. The supernatants were removed from the homogenates and quickly frozen at -80°C until western blot and biochemical analysis. The amount of proteins of supernatants was detected spectrophotometrically using Bradford metho.13,18
Myocardial Malondealdehyde Content
Myocardial malondealdehyde (MDA) as a marker of lipid peroxidation was estimated by measuring thiobarbituric acid-reactive substances (TBARS) in heart homogenates. Briefly, the samples were mixed with 1 mL 10% trichloroacetic acid and 1 mL 0.67% thiobarbituric acid. Then, the samples were heated in boiling water for 15 min, and N-butanol (2:1, v:v) were added to the solution. After centrifugation (900g, 5 min), TBARS were determined from the solution absorbance at 532 nm.19
Myocardial Glutathione Content
Myocardial tissue glutathione (GSH) concentration was quantified using a glutathione reductase-5, 5´-dithiobis (2-nitrobenzoic acid)-based enzymatic recycling assay. To each well of a 96-well plate, 25 µl of standards or supernatant of samples, 25 µl of sample buffer (143 mM NaH2PO4, and 6.3 mM EDTA-Na), and 200 µl of working solution [10 mM glutathione reductase-5,5´-dithiobis (2-nitrobenzoic acid) and 2 mM NADPH in sample buffer] were added. The reaction was initiated by adding 50 µl of 7.2 IU of oxidized glutathione-reductase (Cayman, USA) to each well, and the absorbance change at 405 nm was continuously monitored for 2 min at 9-s intervals with a microplate spectrophotometer. Myocardial GSH concentration was expressed as nanomoles per milligram of protein.
Western Blotting
After electrophoresis of samples, proteins in SDS-gels were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). The transfer was carried out at about 70 mA for 1 hour. Thereafter, the membranes were blocked in 5% skim milk buffer containing 0.1% Tween-20 for 1 hour and then incubated with primary antibodies against the total form of glycogen synthase kinase-3β (GSK-3β), phosphorylated form of GSK-3β at serine-9 (phospho(Ser9)GSK-3β), and β-actin (all antibodies from Cell Signaling, USA) overnight at 4 °C on a shaker. After 3 x 10 min washes with tris buffer saline containing 0.1% Tween-20, membranes were incubated with horseradish peroxidase conjugated (HRP) secondary antibody (Cell Signaling, USA) for 1 hour at RTon a shaker. The membranes were then rinsed at least 3 x 10 min with wash buffer before detection. Blots were then developed using the enhanced chemiluminescence (ECL) method. Following incubation with the ECL reagents, the membranes were exposed to an X-ray hyperfilm inside a hypercassette in darkroom and then the chemiluminescenceof antibody binding was visualized by a visualizing machine.The intensity of protein bands in the blots was digitally quantified using densitometric analysis. To analyze the amount of phosphorylation of GSK-3β in each sample, the ratio of the intensity of phospho(Ser9)GSK-3β to total GSK-3β was calculated and expressed in arbitrary unit (AU).13
Statistical Analysis
All values were expressed as means±SEM. The data were analyzed using one-way ANOVA followed by Tukey's post hoc test. Differences were considered statistically significant when P<0.05.
Results
Risk zone and infarct size
Thirty minutes occlusion of LAD coronary artery produced a similar risk zone in all isolated hearts, so that there were no significant differences in the risk zone among all groups of control or diabetic animals, indicating similarity in induction of ischemia in all hearts (Figure 1). On the other hand, this 30 minutes occlusion and 60 minutes reopening of LAD coronary artery produced an infarct size approximately 41±2.9% of the risk zone in control and 39±3.1% in diabetic groups (Figure 2). The differences between infarct size values of control and diabetic animals was not statistically significant. IPostC significantly reduced the infarct size of non-diabetic control animals to 28±1.9% (P<0.01). However, IPostC could not significantly influence the infarct size in the diabetic rats (35±1.8% vs. 39±3.1%).
Figure 1.
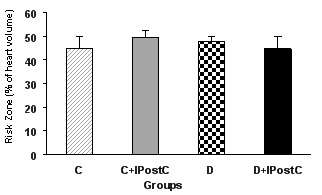
Risk zone (in percent of left ventricle) in control (C) and diabetic (D) animals. IPostC: ischemic postconditioning.
Figure 2.
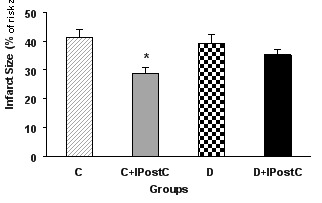
Infarct size (in percent of risk zone) in control (C) and diabetic (D) animals. IPostC: ischemic postconditioning. *P<0.01 vs. group C.
Lipid peroxidation index: MDA level
The level of MDA was measured as an index of lipid peroxidation. The MDA level were significantly reduced by application of IPostC in control animals (P<0.05) whereas, the effect of IPostC was not statistically significant in diabetic animals (Figure 3).
Figure 3.
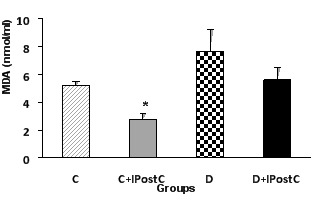
Myocardial MDA activity in control (C) and diabetic (D) animals. IPostC: ischemic postconditioning. *P<0.05 vs. group C.
Glutathione
To determine whether IPostC in control and diabetic hearts may change myocardial GSH, we measured myocardial GSH contents in all experimental groups (Figure 4). IPostC in nondiabetic hearts resulted in a statistically significant elevation in GSH content (27±0.6 vs. 24±0.2 nmol/mg of protein, P<0.05). However, administration of IPostC at the onset of reperfusion in diabetic hearts could not increase GSH content in this condition (26±0.7 vs. 25±0.5 nmol/mg of protein).
Figure 4.
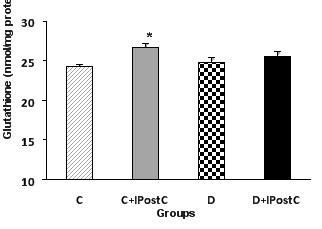
Myocardial glutathione content in control (C) and diabetic (D) animals. IPostC: ischemic postconditioning. *P<0.05 vs. group C.
Total form and the ratio of phosphorylated (at Ser 9) to total form of GSK-3β
For western blotting, the total and phosphorylated (at serine 9) form of GSK-3β were investigated. According to the densitometric analysis, there were no changes in the level of the total form of GSK-3β in control or diabetic animals (Figure 5). Nevertheless, in non-diabetic control animals, the IPostC intervention significantly increased the phosphorylated form of the GSK-3β as compared to those of the control group (P<0.01). However, in diabetic animals, IPostC failed to increase significantly the amount of phosphorylated form of GSK-3β and therefore, there was no difference in the ratio of phosphorylated to total form of GSK-3β between diabetic and diabetic plus IPostC groups (Figure 6).
Figure 5.
The total form of the GSK-3β (AU; arbitrary unit) in the myocardium of control (C) and diabetic (D) animals. GSK-3β: Glycogen Synthase Kinase-3beta, IPostC: ischemic postconditioning.
Figure 6.
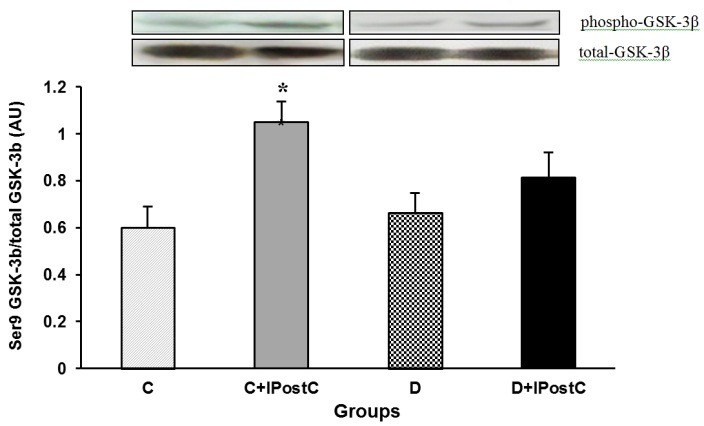
The ratio of phosphorylated(Ser9) to total form of GSK-3β (AU; arbitrary unit) in the myocardium of control (C) and diabetic (D) animals. GSK-3β: Glycogen Synthase Kinase-3beta, Ser9: Serine 9, IPostC: ischemic postconditioning. *P<0.01 vs. group C.
Discussion
In the present study, IPostC, as an effective method of cardioprotection in normal condition without any cardiac risk factors, was failed to induce protection in diabetic hearts. In other words, chronic diabetes (for 8 weeks) prevented the infarct size limiting and protective effect of IPostC in ischemic hearts. The phosphorylation of GSK-3β by IPostC in diabetic hearts was much less than controls one (although this difference was not statistically significant). In addition, IPostC significantly increased tissue content of glutathione and decreased the MDA level in control and healthy hearts; however, it failed to alter the amount of these markers in diabetic group.
IPostC, applied in the protocols of 3 cycles of 30-second reperfusion-ischemia or 6 cycles of 10-second reperfusion-ischemia immediately at the onset of reperfusion, have been showed appropriate protective effects on ischemic heart and caused significant reduction in infarct size.4,11 IPostC is an effective therapeutic strategy which may be used in clinic through inflating and deflating the balloon during angioplasty, and therefore, can be used in patients.5,19 Despite the fact that the most studies in the field of IPostC are performed on healthy animals, ischemic heart disease (IHD) in human patients are mainly resulted from other underlying co-morbidities like diabetes, arthrosclerosis, and hypertension. Diabetes is the most important cardiovascular risk factor that may increase the rate of IHD and prevent cardioprotective effects of therapeutic approaches.2,4
In the present study, chronic diabetes for 8 weeks totally prevented the positive effects of IPostC on reduction of infraction. There are a few studies in this regard; all of them show similar results. Przyklenk reported that 2-week diabetes (although it is not accord with chronic disease as in our study) demolishes the IPostC effects.20 Similarly, it was reported that the protective effect of morphine or sevoflurane are diminished in diabetic and hyperglycemic conditions.21,22 However, why this loss of cardioprotection occurs in diabetic state, is poorly understood yet.
There is not enough study on the mechanisms of interference of diabetes with protective effects of IPostC. Hyperglycemia, increase of oxidative stress and inflammation, and change in the activity of intracellular protein kinases might be considered as the interfering mechanisms, all of which are produced in diabetic conditions23 and may prevent postconditioning effects. GSK-3β is a protein kinase that is active in normal and non-phosphorylated state and can open the mPTP, leading to the release of cytochrome C into the cytosol and starting the apoptosis and oxidative reactions.14
By activating PI3K-Akt signaling pathway, IPostC causes phosphorylation of GSK-3β and inactivates it and therefore, protects the ischemic heart by this way.4,11 In the present study, protective effects of IPostC on non-diabetic hearts accompanied with significant phosphorylation of GSK-3β; however, IPostC failed to phosphorylate and inactivate GSK-3β in diabetic hearts, significantly. Another finding of this study is that the total amount of GSK-3β was the same in diabetic and non-diabetic groups and there were no significant changes among different groups. This result indicates that the chronic diabetes diminishes the power of IPostC to phosphorylate GSK-3β and thus, large quantities of this detrimental protein kinase are remained in its active form in diabetic conditions. Previous studies indicated that the activity of cellular protein kinases changes in diabetic and hyperglycemic conditions. For example, Drenger et al, reported that diabetes causes significant decrease in the amount of phosphorylation and activity of PI3K.22 Therefore, these intracellular changes induced by chronic diabetes act as the interfering factor in the effect of IPostC on phosphorylation of GSK-3β and cardioprotection. As a result, IPostC in diabetic conditions can't phosphorylate and inactivate this protein kinase in a more effective way.
Keeping the GSK-3β in its active form may compromise the processes of cellular damage through the activation of apoptosis and oxidative stress which are mediated by mitochondrial pathways such as mPTP and ATP-sensitive potassium channels or other relative mediators.13,14 In the present study, the amount of glutathione was measured as a strong antioxidant factor and the level of MDA as an index of lipid peroxidation in cardiac tissue of experimental groups. IPostC significantly increased the amount of glutathione and decreased the lipid peroxidation in non-diabetic hearts, however such effects were not observed in diabetic hearts. Postconditioning may attenuate myocardial injury by reducing oxidative stress in vivo in rats and in humans.24-26 It has also been indicated that using exogenous antioxidants or activation of intrinsic antioxidants by postconditioning strategies may cause cardioprotection.27-30 IPostC may decrease the activity of GSK-3β or inactivate it by its phosphorylation, and thereby, increase the cell tolerance to oxidative insult. Therefore, the inability of IPostC to exert cardioprotection in diabetic conditions may be attributed somehow to low activity of antioxidants and/or high peroxidative status which are resulted from diabetes.23 For the better clarification of the postconditioning effects in diabetic hearts, it is suggested to investigate the expression levels of other proteins involved in oxidative stress such as Nrf or eNOS or proteins involved in apoptosis such as BAD/BAX in the future studies.
Conclusion
According to the findings of the present study, chronic diabetes impedes the protective effect of IPostC in diabetic hearts. The low phosphorylation (or low inactivation) of GSK-3β by IPostC, low production of glutathione and high lipid-peroxidation level in diabetic hearts may play the important roles in this loss of cardioprotection.
Acknowledgments
This work was supported by a grant from Research Deputy of Tabriz University of Medical Sciences, Tabriz-Iran.
Ethical Issues
This study (animal handling and all experimentations) was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85-23, revised 1996) and was approved by the local Animal Care Committee.
Conflict of Interest
The authors have no conflicts of interest in regard to this research or its funding.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB. et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittington HJ, Babu GG, Mocanu MM, Yellon DM, Hausenloy DJ. The diabetic heart: too sweet for its own good? Cardiol Res Pract. 2012;2012:845698. doi: 10.1155/2012/845698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA. et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 4.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59(4):418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 5.Kharbanda RK. Cardiac conditioning: a review of evolving strategies to reduce ischaemia-reperfusion injury. Heart. 2010;96(15):1179–86. doi: 10.1136/hrt.2009.179101. [DOI] [PubMed] [Google Scholar]
- 6.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84(2):201–8. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 7.Wagner C, Kloeting I, Strasser RH, Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52(5):430–7. doi: 10.1097/fjc.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- 8.Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol. 2012;11:67. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV. et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 10.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 11.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301(5):H1723–41. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 12.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117(21):2761–8. doi: 10.1161/circulationaha.107.755066. [DOI] [PubMed] [Google Scholar]
- 13.Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol. 2009;604(1-3):111–6. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104(11):1240–52. doi: 10.1161/circresaha.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K. et al. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43(5):564–70. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation--the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86(1):41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109(5):502–11. doi: 10.1161/circresaha.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badalzadeh R, Mohammadi M, Najafi M, Ahmadiasl N, Farajnia S, Ebrahimi H. The additive effects of ischemic postconditioning and cyclosporine-A on nitric oxide activity and functions of diabetic myocardium injured by ischemia/reperfusion. J Cardiovasc Pharmacol Ther. 2012;17(2):181–9. doi: 10.1177/1074248411416118. [DOI] [PubMed] [Google Scholar]
- 19.Yun Y, Duan WG, Chen P, Wu HX, Shen ZQ, Qian ZY. et al. Ischemic postconditioning modified renal oxidative stress and lipid peroxidation caused by ischemic reperfusion injury in rats. Transplant Proc. 2009;41(9):3597–602. doi: 10.1016/j.transproceed.2009.06.203. [DOI] [PubMed] [Google Scholar]
- 20.Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal. 2011;14(5):781–90. doi: 10.1089/ars.2010.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross ER, Hsu AK, Gross GJ. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes. 2007;56(1):127–36. doi: 10.2337/db06-0907. [DOI] [PubMed] [Google Scholar]
- 22.Drenger B, Ostrovsky IA, Barak M, Nechemia-Arbely Y, Ziv E, Axelrod JH. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology. 2011;114(6):1364–72. doi: 10.1097/ALN.0b013e31820efafd. [DOI] [PubMed] [Google Scholar]
- 23.Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. J Pathol. 2013;229(2):232–41. doi: 10.1002/path.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Q, Yang XC, Liu Y, Wang LF, Liu SH, Ge YG. et al. Postconditioning attenuates myocardial injury by reducing nitro-oxidative stress in vivo in rats and in humans. Clin Sci (Lond) 2011;120(6):251–61. doi: 10.1042/CS20100369. [DOI] [PubMed] [Google Scholar]
- 25.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70(2):200–11. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Vaez H, Samadzadeh M, Zahednezhad F, Najafi M. Resistance to reperfusion injury following short term postischemic administration of natural honey in globally ischemic isolated rat heart. Adv Pharm Bull. 2012;2(2):189–96. doi: 10.5681/apb.2012.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badalzadeh R, Yousefi B, Majidinia M, Ebrahimi H. Anti-arrhythmic effect of diosgenin in reperfusion-induced myocardial injury in a rat model: activation of nitric oxide system and mitochondrial KATP channel. J Physiol Sci. 2014;64(6):393–400. doi: 10.1007/s12576-014-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigo R, Prieto JC, Castillo R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: molecular mechanisms and potential clinical applications. Clin Sci (Lond) 2013;124(1):1–15. doi: 10.1042/cs20110663. [DOI] [PubMed] [Google Scholar]
- 29.Ebrahimi H, Badalzadeh R, Mohammadi M, Yousefi B. Diosgenin attenuates inflammatory response induced by myocardial reperfusion injury: role of mitochondrial ATP-sensitive potassium channels. J Physiol Biochem. 2014;70(2):425–32. doi: 10.1007/s13105-014-0320-9. [DOI] [PubMed] [Google Scholar]
- 30.Kumar M, Sharma S, Vasudeva N. In vivo assessment of antihyperglycemic and antioxidant activity from oil of seeds of brassica nigra in streptozotocin induced diabetic rats. Adv Pharm Bull. 2013;3(2):359–65. doi: 10.5681/apb.2013.058. [DOI] [PMC free article] [PubMed] [Google Scholar]


