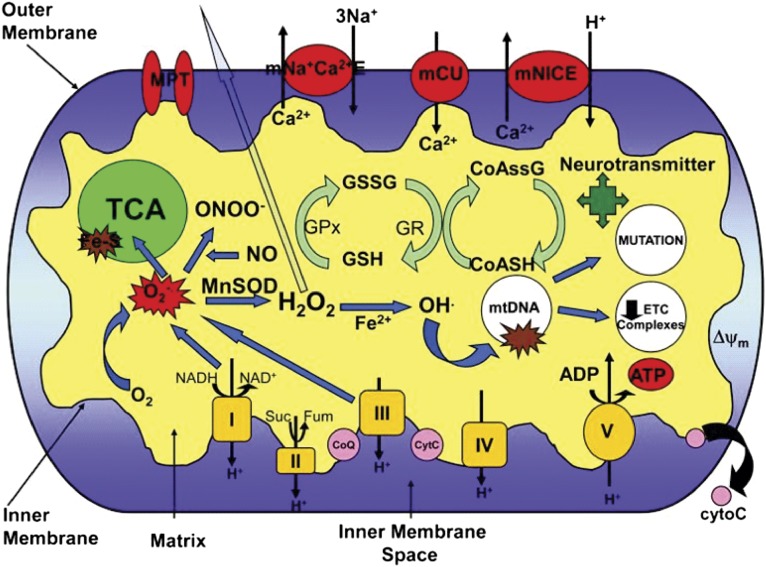
Fig. 3.
Mitochondrial function and neuronal excitability. Various aspects of the mitochondria can lead to impairment of its bioenergetic capacity affecting neuronal excitability, apoptosis, and an increase in seizure susceptibility. O2·, production by complex I and III of the ETC leads to the production of ONOO−, in a reaction with NO, and H2O2 through dismutation by the antioxidant MnSOD (SOD2). H2O2 is membrane-permeable and able to diffuse out of the mitochondria causing widespread oxidative damage. Excessive O2·, production also damages Fe-S-containing enzymes involved in the TCA cycle such as aconitase. OH· can be formed from H2O2 through Fenton chemistry and lead to further oxidative damage of macromolecules such as ETC complexes and mtDNA. Oxidative damage to mtDNA can lead to increased mutation rates and a decrease in ETC subunit expression encoded by the mitochondrial genome. Alterations in the redox status of GSH/GSSG and CoASH/CoASSG can cause an inability to protect against the deleterious effects of ROS. Modification of NT biosynthesis within the mitochondria can affect levels of neuronal excitability/inhibition. Oxidative damage to these targets can result in increased neuronal excitability resulting from decreased ΔΨ and ATP levels affecting the Na+/K+-ATPase and the release of cytochrome C, leading to apoptosis. mNa+C2+E, mitochondrial sodium calcium exchanger; mCU, mitochondrial calcium uniporter; mNICE, mitochondrial sodium independent calcium exchanger; CoASSG, CoA glutathione disulfide; GR, glutathione reductase; GPx, glutathione peroxidase; cytoC, cytochrome C; Suc, succinate; Fum, fumarate. Reprinted with permission (181).