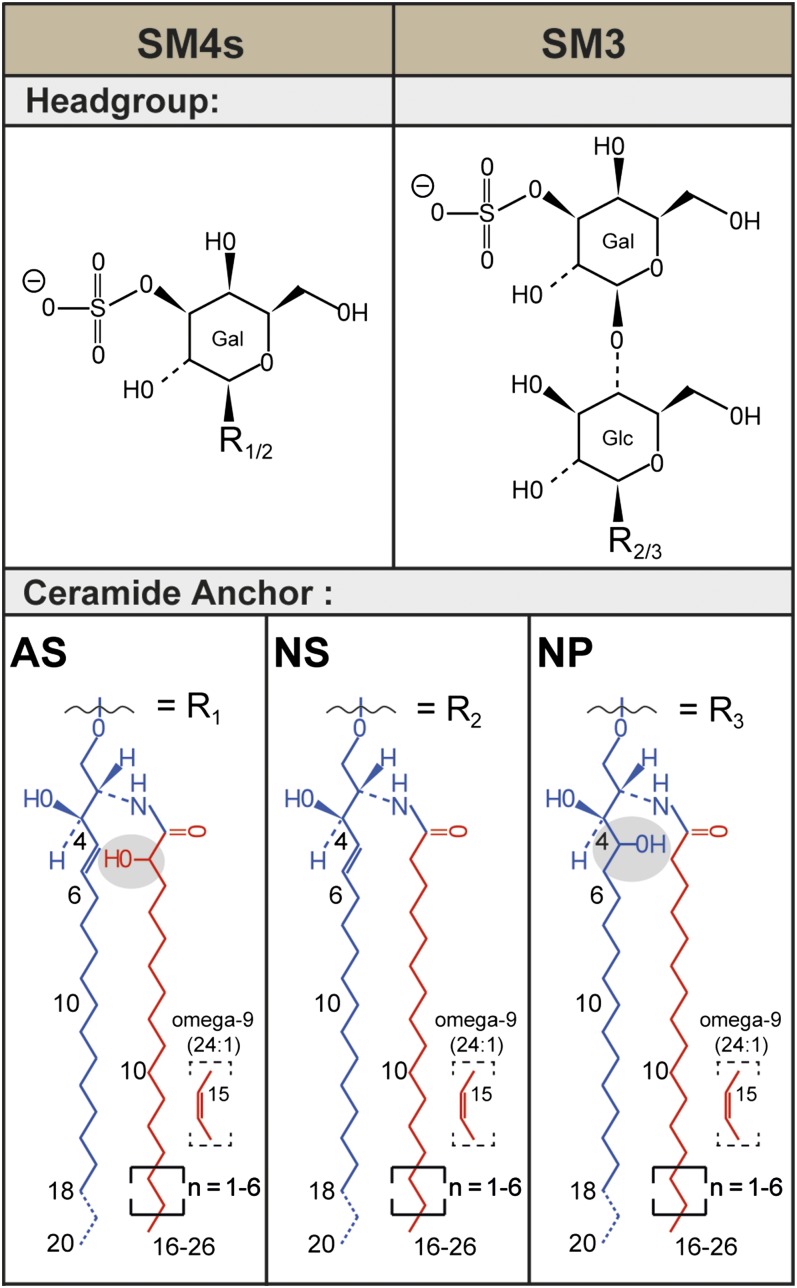
Fig. 1.
Structural diversity of renal sulfatides. Sulfatides are composed of a lipophilic ceramide anchor linked via a β-glycosidic bond to a polar glycan head group, which is sulfated. The most prominent murine and human renal sulfatides are sulfatide SM4s with a monosulfated galactosyl head group and sulfatide SM3 with a monosulfated lactosyl moiety. In mouse kidneys, a variety of ceramide anchors are found in sulfatides. In SM4s, combinations of the major sphingoid base C18-sphingosine (d18:1) with nonhydroxyl acyl chains (NS) and with α-hydroxyl acyl chains (AS) are found. [The ceramide anchor of sphingolipids often is detailed in parentheses following the abbreviation for ceramide (Cer), e.g., Cer(d18:1;16:0) or Cer(t18:0;h16:0). In parentheses, the description of the sphingoid base is followed by the description of the N-linked acyl chain after the semicolon. The sphingoid base is described first with a letter referring to the amount of hydroxy groups within the base (m, mono; d, di; t, tri; and te, tetra). Then the number of C-atoms follows, separated by a colon to the number of double bonds within the sphingoid base. After the semicolon, the fatty acid is described in analogy, but “h” indicates here the presence of one hydroxylation.] SM3 also appears with an NS-ceramide anchor, but, in addition, also anchors combining a C18-phytosphingosine (t18:0) with nonhydroxyl acyl chains (NP) are detected. Ceramide anchors with α-hydroxyl acyl chains are not observed for SM3.