Abstract
Background
Care from higher-volume centers or surgeons has been associated with lower mortality in coronary bypass surgery, but how volume and care quality relate to each other is not well understood.
Objective
To determine how volume and differences in care quality influence outcomes after coronary bypass surgery.
Design
Observational cohort.
Setting
One hundred sixty-four United States hospitals.
Patients
Patients 18 or older who underwent coronary artery bypass grafting between 10/1/2003 and 9/1/2005.
Measurements
Hospital and surgeon case volumes were estimated using our dataset. Quality measures were defined by whether patients did not receive specific medications, and by counting the number of measures missed. We used hierarchical models to estimate effects of volume and quality on mortality and readmission up to 30 days.
Results
After adjustment for clinical factors, lowest surgeon volume and highest hospital volume were associated with lower mortality and lower readmission risk, respectively. Patients who did not receive aspirin (1.89 higher odds, 95% CI 1.65, 2.16) or beta-blockers (1.29 higher odds, 95% CI 1.12, 1.49) had higher mortality, after adjusting for clinical risk factors and case volume; adjusting for individual quality measures did not alter associations between volume and readmission or mortality. However, if no quality measures were missed, mortality at lowest-volume centers (adjusted mortality 1.05%, 95% CI 0.81%, 1.29%), and highest-volume centers (adjusted mortality 0.98%, 95% CI 0.72%, 1.25%) was similar.
Limitations
Because we used administrative data, our quality measures may not replicate measures collected via chart abstraction.
Conclusions
Maximizing adherence to quality measures is associated with improved mortality, independent of hospital or surgeon volume.
Introduction
The volume–outcome relationship — the association between improved surgical outcomes at sites (or from surgeons) that perform a procedure more often –– has become the focus of payor-driven proposals to regionalize care (1). At the same time, many national efforts are focusing on improving surgical care by maximizing adherence to quality of care measures (2).
Focus on quality measures and volume benchmarks has important implications for patients. If quality of care is the most important factor to be weighed when choosing a hospital for surgery, patients can choose a high- or low-volume center as long as these centers have equivalent quality scores. However, if high-volume centers maintain an outcome advantage irrespective of quality of care measures, patients should travel to a regional referral center (3). Limited previous research suggests that once volume is accounted for, quality may have little effect on outcome (4). However, these studies did not use measures of quality that replicated national recommendations.
To explore whether the ‘volume effect’ is explained by care quality and which (volume or quality) is more powerfully associated with patient outcomes, we analyzed data collected from a sample of United States hospitals for adults undergoing coronary artery bypass surgery. Using these data, we first examined the relationship between patient outcomes, hospital case volume, surgeon case volume, and individual quality measures after accounting for clinical risk factors. When then tested how quality and volume affect each other. Finally, we examined whether meeting all or just some quality measures influenced mortality and calculated estimated mortality rates.
Methods
Sites and subjects
Our data were collected on 81,289 patients cared for by 1,451 surgeons at 164 hospitals participating in Perspective (Premier Inc., Charlotte, North Carolina), a voluntary, fee-supported database developed for measuring quality and health care utilization, which we have used in previous research (5–7).
In addition to standard hospital discharge file data, Perspective contains a date-stamped log of all materials (e.g. serial compression devices used to prevent venous thromboembolism), and medications (e.g. beta-blockers) charged for during hospitalization. Perspective charge data are collected electronically from participating sites and audited regularly as part of Premier efforts to ensure data validity.
Perspective sites are generally representative of the US hospital population in that they are predominantly small to mid-size, non-teaching facilities, which serve a largely urban patient population. Perspective sites also have similar performance on publicly reported quality measures.
Patients in our analysis were admitted between 10/1/2003 and 9/1/2005, were age 18 years or older, and had coronary bypass grafting (CABG) as their principal procedure (defined by International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code). The institutional review board at University of California, San Francisco approved our study. The funder (California HealthCare Foundation) had no role in the development or execution of the study, or preparation of the manuscript.
Data
In addition to patient age, sex, race or ethnicity, insurance information, and principal diagnosis, we classified comorbidities using the method of Elixhauser (8). Data regarding in-hospital deaths and readmission at the index hospital at 30 days were obtained from the Perspective discharge file. In addition, the database contained information about hospital size, teaching status, and location.
Definition of volume measures
Because some hospitals in our cohort did not contribute data for the entire study period, we estimated the annual case volume by dividing each hospital’s or surgeon’s observed patient count by the total number of months that the hospital or surgeon contributed patients to the dataset. These “annualized” volumes were then divided into quartiles as done in previous work (4, 9–11).
Definition of missed quality measures
Using charge data, we translated recommendations from the Surgical Care Improvement Project (SCIP) (2) and American Heart Association/American College of Cardiologists Guidelines for secondary prevention of coronary artery disease among patients undergoing coronary bypass surgery (12) into a series of dichotomous quality measures (Table 2). These measures, many of which were also included in recently published recommendations (13), included whether antimicrobials were used to prevent surgical site infection on the operative day, whether that antimicrobial was appropriate, whether serial compression devices were used to prevent venous thromboembolism on the operative day, and whether aspirin, beta-blockers, or statin lipid-lowering drugs were administered in the two days following surgery. Other SCIP measures (such as those related to hair removal, glucose control, and discontinuing antimicrobials at 48 hours) cannot be detected in Perspective data and were not targeted.
Table 2.
Definitions and descriptive statistics for quality measures
| Quality Measures | Measure definition | Exclusion criteria | N (%) |
|---|---|---|---|
| No use of serial compression devices in first 2 days |
No charges for serial compression devices in the 2 calendar days following surgery |
None | 62231 (77%) |
| No statins in first 2 days after surgery | No charge for ‘statin’ lipid lowering drug in 2 calendar days following surgery (e.g. lovastatin, pravastatin, atorvastatin, etc.) |
Principal or secondary diagnosis code for liver disease, cirrhosis, myopathy. |
45579 (56%) |
| Inappropriate choice of prophylactic antimicrobials |
Use of antimicrobial not on approved list | Principal or secondary diagnosis code for preexisting infection, as defined by Surgical Care Improvement Project (SCIP) |
29486 (36%) |
| No prophylactic antibiotics | No charges for antimicrobials on approved list | Principal or secondary diagnosis code for preexisting infection, as defined by Surgical Care Improvement Project (SCIP) |
5167 (6%) |
| No aspirin in first 2 days after surgery | No charge for aspirin in 2 days after surgery | Principal or secondary diagnosis code for cerebrovascular hemorrhage, gastrointestinal bleeding, factor deficiencies, platelet disorders. |
28183 (35%) |
| No beta-blockers in first 2 days after surgery |
No charges for adrenergic blocking agents in 2 days after surgery |
Principal or secondary diagnosis code for conduction system disorder, hypotension, sepsis, congestive heart failure, or bradycardia. |
15998 (20%) |
Because inpatient diagnosis codes cannot reliably distinguish between complications and preexisting conditions (14–16), we measured the proportion of ideal candidates for each care process who failed to receive them — a missed quality measure. For example, we considered beta-blocker use ‘missed’ if a patient did not receive the drug and did not have ICD-9 coded principal or secondary diagnosis of hypotension, heart block, or congestive heart failure recorded in their record. To provide a more sensitive measure of system-level ability to provide reliable care (17), we also counted the number of quality measures missed during hospitalization.
Analysis
We first described study patients and hospitals using univariable methods. Multivariable alternating logistic models (15) (SAS PROC GENMOD) were then used to account for clustering of effects attributable to surgeons and hospitals who had more than patient in our dataset; results are reported as odds ratios with 95% confidence intervals. Models were constructed using a combination of automated and manual variable selection methods. Volume and quality measures were entered manually, while additional covariates (confounding factors) were selected for inclusion if they were associated with the outcome at p<0.01, if including them changed estimates for the primary predictors by more than 10%, or for face validity.
The goal of our analysis was to determine whether our key predictors — hospital volume, surgeon volume, individual quality measures, or overall care quality (the total number of individual measures missed) — were associated with our key outcomes (mortality and readmission) after adjustment for confounding patient or site-related factors (such as patient comorbidity or hospital teaching status). To achieve this goal, we performed our analyses in stages, first by testing individual predictors in models singly and adjusting only for confounding patient and site factors. Our next modeling stage assessed whether effects of hospital or surgeon volume were mediated by quality measures, by constructing models adjusting for hospital volume, and surgeon volume, and quality measures, as well as confounding factors. We then compared our first and second set of models for attenuation of the adjusted odds-ratios toward 1.0 as a way to determine mediation of effects related to volume or quality of care (individual quality measures or the overall quality measure). Finally, to better display the absolute differences in mortality attributable to volume and overall quality, we calculated adjusted mortality rates.
To assess for collinearity between our key predictors (hospital volume, surgeon volume, and quality measures), we examined Pearson correlations between them; these analyses gave little evidence for collinearity (all correlations <0.3). In addition, we examined models including only subsets of these variables and found no evidence for instability. Finally, we checked for interactions between volume and quality measures, and found no statistically significant modification of effects. All analyses were carried out using SAS version 9.1 (SAS Institute, Inc. Cary, NC).
Results
Patient characteristics (Table 1)
Table 1.
Characteristics of patients (n=81289)
| Value | |
|---|---|
| Patient age (Mean, standard deviation) | 65.0 (10.9) |
| Male (n, %) | 58398 (72%) |
| Race (n, %) | |
| White | 61621 (76%) |
| Other | 11434 (14%) |
| Black | 5500 (7%) |
| Hispanic | 2734 (3%) |
| Marital status (n, %) | |
| Married | 51094 (63%) |
| Single | 8646 (11%) |
| Widowed | 8439 (10%) |
| Other | 6899 (8%) |
| Divorced | 6211 (8%) |
| Primary payor (n, %) | |
| Medicare | 43164 (53%) |
| Managed Care | 21987 (27%) |
| Indemnity | 8177 (10%) |
| Medicaid | 3614 (4%) |
| Uninsured | 2575 (3%) |
| Other | 1057 (1%) |
| Capitated | 715 (1%) |
| Discharge status (n, %) | |
| To home | 43588 (54%) |
| Home health care | 24444 (30%) |
| Skilled nursing facility | 8028 (10%) |
| Rehabilitation | 2574 (3%) |
| Death in hospital | 1738 (2%) |
| Transfer | 399 (0.5%) |
| Other | 443 (0.5%) |
| Hospice | 75 (0.1%) |
| Any intensive care unit charges (n, %) | 60392 (74%) |
| All patient refined (APR)™ diagnosis-related group risk of mortality (n, %) | |
| 1 | 8702 (11%) |
| 2 | 40789 (50%) |
| 3 | 23747 (29%) |
| 4 | 8051 (10%) |
| All patient refined (APR)™ diagnosis-related group severity score (n, %) | |
| 1 | 27388 (34%) |
| 2 | 32065 (39%) |
| 3 | 15883 (20%) |
| 4 | 5953 (7%) |
| Comorbidities (n, %) | |
| Hypertension | 58492 (72%) |
| Diabetes without chronic complications | 25423 (31%) |
| Chronic pulmonary disease | 18974 (23%) |
| Fluid and electrolyte disorders | 12815 (16%) |
| Deficiency anemia | 11981 (15%) |
| Obesity | 11636 (14%) |
| Peripheral vascular disease | 11034 (14%) |
| Coagulopathy | 6335 (8%) |
| Hypothyroidism | 6038 (7%) |
| Diabetes with chronic complications | 4623 (6%) |
| Renal failure | 4308 (5%) |
| Depression | 3781 (5%) |
| Other neurological disorders | 1882 (2%) |
| Alcohol abuse | 1663 (2%) |
| Rheumatoid arthritis or collagen vascular disease | 1191 (1%) |
| Psychoses | 1006 (1%) |
| Paralysis | 949 (1%) |
| Solid tumor without metastasis | 918 (1%) |
| Congestive Heart Failure | 443 (0.5%) |
| Internal mammary graft not used (n, %) | 9938 (12%) |
| Characteristics of site of care | |
| Teaching hospital (n, %) | 30295 (37%) |
| Urban hospital | 76079 (94%) |
| Rural hospital | 5210 (6%) |
| Region (n, %) | |
| South | 46768 (58%) |
| Midwest | 14082 (17%) |
| Northeast | 11201 (14%) |
| West | 9237 (11%) |
| Number of beds (n, %) | |
| 100–199 | 2952 (4%) |
| 200–299 | 7469 (9%) |
| 300–399 | 16678 (21%) |
| 400–499 | 13373 (16%) |
| >=500 | 40817 (50%) |
| Outcomes (n, %) | |
| Mortality up to 30 days | 1825 (2%) |
| Readmission up to 30 days | 8653 (11%) |
81,289 patients underwent coronary artery bypass grafting at one of the study sites between 10/1/2003 and 9/30/2005. Mean age of patients was 65.0 years (standard deviation 10.9 years), and 72% were men. Most were white, married, and had Medicare insurance. The most common comorbidities in our cohort were hypertension (72%), diabetes without chronic complications (31%), and chronic obstructive pulmonary disease (23%). Most received care at nonteaching hospitals in the South. Two percent (1825/81289) of patients died, and 11% were readmitted in 30 days.
Quality measures
Definition of individual quality measures is presented in Table 2. The proportion of patients for whom individual quality measures were missed varied widely. Most (77%) did not have charges for serial compression devices, but few did not receive a beta-blocker (22%), and few had no antimicrobial charges on the operative day (6%). Few patients (12%) had no missed quality measures and 44% missed 3 or more. Among patients with 1–2 missed care measures, failure to receive a serial compression device occurred among 70%, 33% received no statins, 8% received no aspirin, 29% received inappropriate antibiotics, and 14% received no beta-blocker.
Hospital and surgeon volume and rates of missed quality measures (Table 3)
Table 3.
Number of hospitals or providers and missed quality measures per quartile of patient volume
| Number of patients |
Number of hospitals or surgeons |
Median volume (25th-75th interquartile range) |
Number of deaths (%) |
Number of readmissions (%) |
Mean number of missed quality measures (standard deviation)* |
|
|---|---|---|---|---|---|---|
| Hospital annual | ||||||
| volume | ||||||
| 1st quartile (lowest volume) | 20358 | 85 | 142 (104, 175) | 489 (2.4%) | 2311 (11.4%) | 2.29 (1.40) |
| 2nd quartile | 20089 | 40 | 244 (217, 266) | 392 (2.0%) | 2179 (10.9%) | 2.21 (1.35) |
| 3rd quartile | 20491 | 26 | 373 (340, 423) | 487 (2.4%) | 2307 (11.3%) | 2.11 (1.27) |
| 4th quartile (highest volume) | 20351 | 13 | 744 (548, 1166) | 457 (2.3%) | 1856 (9.1%) | 2.56 (1.53) |
| Surgeon annual | ||||||
| volume | ||||||
| 1st quartile (lowest volume) | 20331 | 1143 | 40 (24, 53) | 534 (2.6%) | 2208 (10.9%) | 2.24 (1.41) |
| 2nd quartile | 20350 | 150 | 77 (71, 86) | 457 (2.3%) | 2193 (10.8%) | 2.18 (1.42) |
| 3rd quartile | 20402 | 96 | 108 (101, 117) | 414 (2.0%) | 2180 (10.7%) | 2.37 (1.36) |
| 4th quartile (highest volume) | 20206 | 62 | 158 (142, 193) | 420 (2.1%) | 2072 (10.3%) | 2.40 (1.40) |
P for trend in number of missed quality measures <0.0001 for both hospital and surgeon annual volume.
The majority of hospitals (85 hospitals, 51%) and surgeons (1143 surgeons, 78%) were lowest-volume providers. Hospital volume ranged from 142 (IQR 104, 175) in the lowest volume quartile to 744 per year (IQR 548, 1166) in the highest. Surgeon volume ranged from 40 patients per year (IQR 24, 53) in the lowest volume quartile, to 158 (IQR 142, 193) in the highest. Mortality and readmission rates were similar across quartiles of hospital and surgeon volume. Although univariable tests for linear trend were statistically significant, there were very small absolute differences in rates of missed quality measures across hospital or surgeon volume quartiles. In fact, higher volume hospitals or surgeons tended to have more missed quality measures than smaller ones.
Effects of volume on outcomes ( Table 4)
Table 4.
Hospital and surgeon volume and care outcomes (n = 81289 patients)
| 30-day mortality (1825 deaths) | Readmission (8653 readmissions) | |||||
|---|---|---|---|---|---|---|
| Value | Unadjusted Odds Ratio (95% CI) |
Adjusted odds - Adjusted for confounders only (95% CI) |
Adjusted* Odds Ratio - Adjusted for individual quality measures and confounders (95% CI) |
Unadjusted Odds Ratio (95% CI) |
Adjusted odds - Adjusted for confounders only (95% CI) |
Adjusted** Odds Ratio - Adjusted for individual quality measures (95% CI) |
| Hospital annual | ||||||
| volume | ||||||
| 1st quartile (lowest volume) | 1.07 (0.94, 1.22) | 1.23 (0.98, 1.55) | 1.14 (0.89, 1.45) | 1.28 (1.20, 1.36) | 1.24 (1.08, 1.42) | 1.22 (1.06, 1.41) |
| 2nd quartile | 0.87 (0.76, 0.99) | 1.10 (0.86, 1.41) | 1.12 (0.87, 1.44) | 1.21 (1.14, 1.29) | 1.19 (1.03, 1.36) | 1.16 (1.01, 1.33) |
| 3rd quartile | 1.06 (0.93, 1.21) | 1.22 (0.97, 1.53) | 1.28 (1.01, 1.61) | 1.26 (1.19, 1.35) | 1.22 (1.06, 1.40 | 1.21 (1.05, 1.39) |
| 4th quartile (highest volume) | Referent | Referent | Referent | Referent | Referent | Referent |
| Surgeon annual | ||||||
| volume | ||||||
| 1st quartile (lowest volume) | 1.27 (1.12, 1.45) | 1.26 (1.05, 1.51) | 1.26 (1.02, 1.56) | 1.07 (1.00, 1.14) | 1.06 (0.98, 1.15) | 1.02 (0.94, 1.11) |
| 2nd quartile | 1.08 (0.95, 1.24) | 1.11 (0.92, 1.33) | 1.11 (0.90, 1.36) | 1.06 (0.99, 1.13) | 1.09 (1.01, 1.17) | 1.05 (0.96, 1.15) |
| 3rd quartile | 0.98 (0.85, 1.12) | 1.04 (0.88, 1.23) | 1.03 (0.85, 1.24) | 1.05 (0.98, 1.12) | 1.04 (0.96, 1.13) | 1.03 (0.95, 1.12) |
| 4th quartile (highest volume) | Referent | Referent | Referent | Referent | Referent | Referent |
CI – Confidence interval
Confounders included in mortality models: age, gender, diagnosis-related group predicted mortality, congestive heart failure, hypertension, neurological disorders, diabetes with complications, renal failure, coagulopathy, deficiency anemia, and whether or not an internal mammary graft was used during the procedure.
Confounders included in readmission models: age, gender, race, insurance type, admission status, diagnosis-related group predicted mortality, chronic obstructive lung disease, peripheral vascular disease, diabetes, diabetes with complications, renal failure, electrolyte disorders, deficiency anemia, psychoses, depression, and geographic region.
In unadjusted analyses, there were no consistent associations between surgeon or hospital volume and mortality or readmission. After adjusting for confounding patient and hospital factors, there continued to be little consistent gradient in association between hospital volume and odds for mortality, although hospital volume remained associated with lower odds for readmission. Compared to patients who received care from highest volume surgeons, patients whose operation was performed by a low-volume surgeon had higher odds for death. After adjustment for individual quality measures, no significant changes in these findings were noted, suggesting that the effect of surgeon volume on patient outcomes was independent of hospital factors, quality of care, or confounding factors.
Effects of individual and overall quality on outcomes (Table 5)
Table 5.
Quality measures and care outcomes (n = 81289 patients)
| 30-day mortality (1825 deaths) | Read mission (8653 readmissions) | |||||
|---|---|---|---|---|---|---|
| Value | Unadjusted Odds Ratio (95% CI) |
Adjusted* odds ratio - Adjusted for confounders only (95% CI) |
Adjusted* Odds Ratio - Adjusted for volume measures and confounders (95% CI) |
Unadjusted Odds Ratio (95% CI) |
Adjusted** odds - Adjusted for confounders only (95% CI) |
Adjusted** Odds Ratio - Adjusted for volume measures and confounders (95% CI) |
| Individual measures | ||||||
| No aspirin | 1.17 (1.06, 1.29) | 1.90 (1.66, 2.16) | 1.89 (1.65, 2.16) | 0.91 (0.87, 0.95) | 1.01 (0.94, 1.08) | 1.00 (0.94, 1.06) |
| No beta-blocker | 0.82 (0.72, 0.93) | 1.29 (1.12, 1.48) | 1.29 (1.12, 1.49) | 0.83 (0.78, 0.88) | 0.95 (0.89, 1.01) | 0.96 (0.90, 1.02) |
| No statin | 1.23 (1.12, 1.35) | 1.07 (0.95, 1.20) | 1.07 (0.95, 1.21) | 0.95 (0.91, 0.99) | 0.97 (0.91, 1.04) | 0.96 (0.91, 1.02) |
| No prophylactic antibiotics |
1.12 (0.94, 1.35) | 1.25 (0.77, 2.00) | 1.25 (0.79, 2.00) | 0.82 (0.74, 0.91) | 0.87 (0.76, 1.00) | 0.90 (0.80, 1.01) |
| Inappropriate antibiotics | 2.05 (1.86, 2.25) | 0.66 (0.58, 0.74) | 0.66 (0.58, 0.75) | 1.26 (1.20, 1.32) | 1.10 (1.05, 1.15) | 1.12 (1.08, 1.17) |
| No serial compression device |
1.22 (1.09, 1.37) | 1.00 (0.82, 1.22) | 1.00 (0.83, 1.22) | 1.01 (0.95, 1.06) | 0.99 (0.90, 1.09) | 1.01 (0.93, 1.09) |
|
Number of measures missed* |
||||||
| 0 missed | Referent | Referent | Referent | Referent | Referent | Referent |
| 1 missed | 1.68 (1.35, 2.10) | 1.05 (0.79, 1.37) | 1.05 (0.80, 1.38) | 1.10 (1.01, 1.20) | 1.00 (0.92, 1.10) | 1.01 (0.92, 1.10) |
| 2 missed | 1.58 (1.28, 1.95) | 1.15 (0.87, 1.53) | 1.15 (0.87, 1.53) | 1.09 (1.01, 1.18) | 0.99 (0.91, 1.08) | 1.00 (0.92, 1.09) |
| 3 missed | 2.43 (1.99, 2.98) | 1.54 (1.20, 1.98) | 1.54 (1.20, 1.98) | 1.08 (0.99, 1.17) | 0.99 (0.90, 1.10) | 1.00 (0.91, 1.10) |
| 4 or more | 2.37 (1.92, 2.92) | 1.62 (1.23, 2.14) | 1.63 (1.24, 2.15) | 1.03 (0.95, 1.13) | 0.98 (0.88, 1.10) | 1.02 (0.93, 1.13) |
CI – Confidence interval.
Number of measures missed: 9378 patients (12%) had no missed measures, 14884 (18%) had one missed, 20534 (25%) had 2 missed, 21232 (26%) had 3 missed, and 15261 (18%) had 4 or more missed.
Confounders included in mortality models: age, gender, diagnosis-related group predicted mortality, congestive heart failure, hypertension, neurological disorders, diabetes with complications, renal failure, coagulopathy, deficiency anemia, and whether or not an internal mammary graft was used during the procedure.
Confounders included in readmission models: age, gender, race, insurance type, admission status , diagnosis-related group predicted mortality, chronic obstructive lung disease, peripheral vascular disease, diabetes, diabetes with complications, renal failure, electrolyte disorders, deficiency anemia, psychoses, depression, and geographic region.
Individual quality measures had varied associations with mortality and readmission risk. Patients who did not receive aspirin (1.89 higher odds, 95% CI 1.65, 2.16) or beta-blockers (1.29 higher odds, 95% CI 1.12, 1.49) had higher mortality, after adjusting for clinical risk factors and case volume; only inappropriate antimicrobial use was associated with differences in readmission risk. For either outcome, adjusting for individual quality measures did not alter any associations with surgeon or hospital case volume.
In contrast, there were strong associations between the number of quality measures missed and mortality. After adjustment, patients who missed 3 measures (adjusted odds ratio 1.54, 95% CI 1.20, 1.98) and those who missed 4 or more (adjusted odds ratio 1.63, 95% CI 1.24, 2.15) had higher odds for death compared to those who missed no quality measures. The number of missed quality measures was not associated with readmission risk.
Overall care quality, case volume, and patient outcomes (Figures 1 and 2)
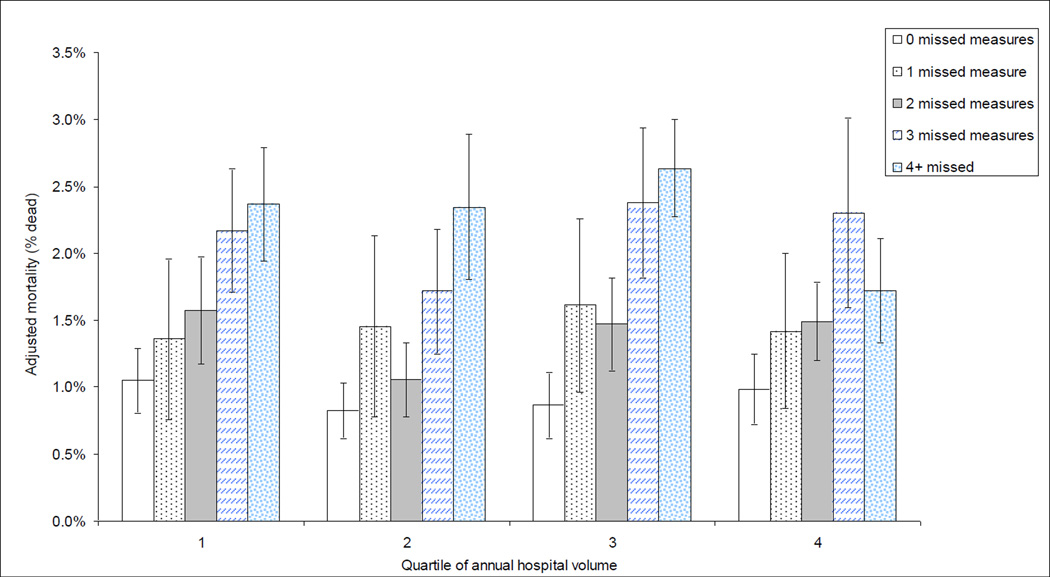
Figure 1. Effects of hospital case volume and missed quality measures on mortality.
Adjusted mortality of patients undergoing coronary bypass surgery by quartile of hospital volume and count of missed quality measures. A strong association between the number of quality measures missed and mortality across all quartiles of volume is observed, with mortality similar across quartiles of hospital case volume if no quality measures are missed. Models adjusted for age, gender, DRG predicted mortality, congestive heart failure, hypertension, neurological disorders, diabetes with complications, renal failure, coagulopathy, deficiency anemia, and whether or not an internal mammary graft was used during the procedure, the volume and number of missed quality measures as well as for the interaction between volume and number of missed quality measures.
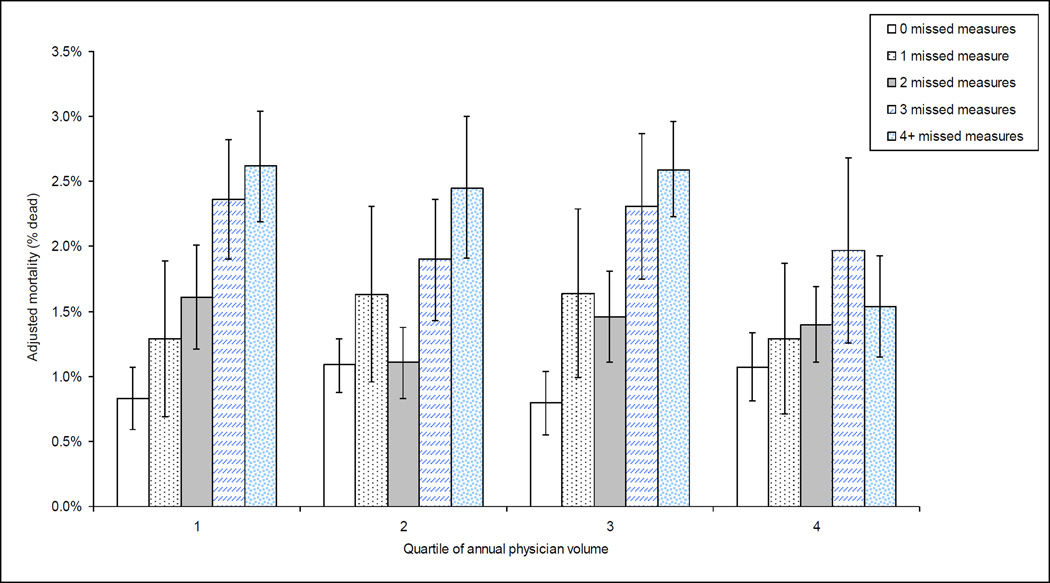
Figure 2. Effects of surgeon case volume and missed quality measures on mortality.
Adjusted mortality of patients undergoing coronary bypass surgery by quartile of surgeon volume and count of missed quality measures. As for hospital volume, strong association between the number of quality measures missed and mortality across all quartiles of surgeon volume is observed, with mortality similar even for lowest volume surgeons if no quality measures are missed. Models adjusted for age, gender, DRG predicted mortality, congestive heart failure, hypertension, neurological disorders, diabetes with complications, renal failure, coagulopathy, deficiency anemia, and whether or not an internal mammary graft was used during the procedure, the volume and number of missed quality measures as well as for the interaction between volume and number of missed quality measures.
When we estimated mortality (adjusted for age, gender, DRG predicted mortality, congestive heart failure, hypertension, neurological disorders, diabetes with complications, renal failure, coagulopathy, deficiency anemia, and whether or not an internal mammary graft was used during the procedure), mortality was negligibly associated with hospital volume but strongly influenced by the number of quality measures missed (Figure 1). In the lowest volume quartile, adjusted mortality rates rose from 1.05% (95% CI 0.81%, 1.29%) if none were missed to 2.37% (95% CI 1.94%, 2.79%) if 4 or more were missed. In fact, if no quality measures were missed, lowest-volume (adjusted mortality 1.05%, 95% CI 0.81%, 1.29%) and highest-volume hospitals had similar mortality.
Similar findings were seen in analyses of mortality by quartile of surgeon volume and the number of missed quality measures (Figure 2). The association between surgeon volume and outcome was inconsistent, but mortality among patients with no missed quality measures rose from 0.83% (95% CI 0.39%, 1.02%) in the lowest quartile of surgeon volume to 1.07% (95% CI 0.78%, 1.36%) in the highest quartile.
Secondary analyses
To test the robustness of our results, we conducted analyses in which we fit models including covariates with p-values less than 0.20, rather than p<0.01. Results from these analyses also did not demonstrate consistent associations between volume and outcome. Nor did less parsimonious models change the associations between overall care quality and mortality. We then examined the influence of interactions between volume measures and factors that might potentially represent shifting of complex cases to higher volume hospitals or surgeons. Specifically, we checked for interactions between the volume variables and APR-DRG risk of mortality and APR-DRG severity of illness. These interaction terms were not statistically significant, and inclusion of these terms in our models did not alter our results. There was no statistically significant interaction between hospital or surgeon volume and missed quality measures (p>0.5 for both). This finding suggests that quality and volume attributed differences to outcomes independently.
Discussion
In this large cohort of patients undergoing coronary artery bypass surgery, we found that hospital and surgeon volume had few consistent associations with mortality or risk for readmission after cardiac surgery. Likewise, individual quality of care measures had a similarly inconsistent pattern of association with clinical outcomes, and adjusting for quality of care differences or patient characteristics did not reveal an association between volume measures and our outcomes. However, overall adherence to quality measures was strongly associated with differences in mortality regardless of hospital or surgeon volume. Because improving quality of care at hospitals is potentially more feasible than increasing case volume, these results may have substantial implications for patients undergoing coronary artery bypass surgery.
The relationship between higher volume of care and better outcomes of cardiac surgery is well recognized (11, 18–20) and has been endorsed as a way for purchasers to identify preferred sites and improve patient outcomes (1)—an approach aptly termed ‘follow the crowd’ (21). However, regionalization of services poses practical problems for hospitals that need to try to meet volume standards and for patients who need to travel for surgery and perioperative care (22, 23). In addition, the evidence for volume benchmarks’ ability to accurately identify ‘best’ sites has limitations (3, 24, 25).
We did note an association between lowest volume surgeons and higher mortality, and an association between higher hospital volume and lower risk for readmission. However, the strength of the volume– outcomes associations we observed is weaker and less consistent than in other studies (11, 18–20). While it seems likely that higher case volume remains an important route to improved outcomes, a number of interrelated trends may be affecting the volume–outcome relationship in cardiac surgery (26). For example, emergence of improved techniques in interventional cardiology, the push to disseminate best practices across sites, and public reporting of mortality rates (27) are providing a number of confounding factors that may influence outcomes independent of case-volume. In addition, shorter length of stay and shifting care to post-discharge settings may also limit the association between shorter-term mortality and volume. Indeed, recent work has suggested that higher surgeon and hospital volume are associated with better longer term outcomes of cardiac surgery (28).
Guidelines such as those we used to develop our study’s quality measures (2, 12) represent care practices that should be followed regardless operative volume. Of the measures tested, not receiving beta blockers or aspirin was associated with higher mortality and not receiving a statin lipid-lowering drug barely missed tests of statistical significance, suggesting the rationale for these measures may be sound. However, after adjustment, getting an incorrect antimicrobial was associated with lower mortality, a finding which may indicate gaps in documentation (i.e. lack of documentation for why antimicrobials might have been continued appropriately). Our data are consistent with previous evidence suggesting that performance on publicly reported quality measures explains only a small portion of differences in patient outcomes (29); early experience with Surgical Care Improvement Project measures in colorectal surgery has not seen a relationship between quality measures and improved outcomes (30).
Overall quality — in our study, not missing any quality measures — is thought to be a measure of a system’s ability to deliver all aspects of care reliably (17), whereas individual practices may be divided among team members (29, 31). Our results support publications that endorse maximizing overall quality (32) as a way to compel outcome improvement by compelling the development of systems which are highly reliable – that is, reliable systems provide care which is consistent from patient to patient because the system compels consistency rather than being dependent on individual team members’ individual efforts. Indeed, we saw strong trends in outcome even though our overall quality measurement included individual measures with weak, or reversed, associations with mortality. Refining this listing to just measures with a beneficial effect on mortality or reweighting them (another proposed method for maximizing impact of quality reporting) would likely magnify the importance of overall quality in identifying optimal systems. Maximizing care quality at low-volume hospitals (over regionalization alone) (33) has advocates; our results would support this approach. Overall care quality was not associated with readmission risk — a particularly striking finding when juxtaposed with the association between quality and 30-day mortality. While our data do not allow us to directly test these hypotheses, it is possible that readmission risk is more influenced by care delivered at discharge, such as care planning or the presence of support during the post-discharge time period, whereas in-hospital mortality is dependent more on decisions and care provided earlier in the hospital stay (captured in our data), such as medications.
Our study has a number of limitations: (i) Because we used administrative data, we cannot easily distinguish complications from preexisting disease. However, we constructed the quality measures to focus on patients who had no documented contraindications, and we did not use comorbidities to define outcomes. (ii) Our quality measures focus primarily on inpatient medications and cannot distinguish continuation of home medications from initiation of medications in hospital. This factor may be influencing the associations between mortality and aspirin, beta-blockers, and statins, but is less likely to affect antimicrobial or serial compression device use. (iii) Our quality measures were collected from electronic billing systems rather than chart abstraction, and have not been validated in a scientific study. However, because Premier’s business model focuses on provision of accurate benchmarking data to their members, all charge and diagnosis data are regularly audited for accuracy. (iv) Our mortality and readmission outcomes focus on events taking place at only the index hospital and may miss these events if they took place elsewhere. Nor did we include other clinical outcomes of interest in cardiac surgery, such as relief of angina or later cardiac events (28). Lack of these clinical outcomes prompted us to use readmission as a proxy for short-term adverse events. (v) As an observational study, the results are subject to biases related to nonrandom assignment of patients to receive medications or devices, as well as documentation biases described. However, secondary analyses, including adjustment for hospital-level likelihood of receipt of quality measures, did not suggest this bias was a substantial threat. (vi) We examined only one surgical procedure, and our results cannot be extrapolated to other high-risk surgeries such as cancer surgery, where volume is thought to be an important predictor of outcome. Although Premier hospitals are similar to other US centers in terms of size, teaching status, and location, they may differ from non-Premier sites in subtle ways not captured in our data. Nonetheless, previous research in Premier sites has produced results useful to policymakers (34). (vii) While we constructed our volume measures to be consistent with those employed in previous work, it is possible that they do not adequately represent expertise accrued if low volume surgeons were performing other complex surgeries frequently. (viii) It is likely that some surgeries in our dataset were at least partially performed by fellows or residents. To address this potential concern, we did adjust for whether the surgery was performed at a teaching hospital.
Our study represents an important view of how case volume, care quality, and outcomes of care are related. Although efforts to encourage patients to ‘follow the crowd’ to a higher volume site or surgeon (or at least avoid lowest volume ones) may be useful, our results suggest that volume alone may be of less importance in the current era. In contrast, our results suggest that efforts to increase the overall quality of care so that patients can ‘shop for the best’ provides a higher likelihood of benefits, and represent an approach that could be implemented wherever coronary artery bypass surgery is performed, regardless of surgeon or hospital volume.
Acknowledgements
We would like to acknowledge Erin Hartman MS for her expert editorial assistance, as well as Denise Remus MD and Kathy Belk for their work in assembling the dataset used for this analysis. Dr Auerbach had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding:
Supported by Grant #05-1755 from the California HealthCare Foundation
Dr. Auerbach was also supported by a K08 Patient Safety Research and Training Grant (K08 HS11416-02) from the Agency for Healthcare Research and Quality during the execution of this project.
Footnotes
Disclosures:
The authors declare no conflicts of interest in relationship to this article
References Cited
- 1.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135(6):569–575. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed February 9, 2009];Surgical Care Improvement Program. at http://www.medqic.org/dcs/ContentServer?cid=1122904930422&pagename=Medqic%2FContent%2FParentShellTemplate&parentName=Topic&c=MQParents.
- 3.Dimick JB, Finlayson SR, Birkmeyer JD. Regional availability of high-volume hospitals for major surgery. Health Aff (Millwood) 2004;(Suppl Web Exclusives) doi: 10.1377/hlthaff.var.45. VAR45-53. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106(11):2476–2481. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 5.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291(17):2092–2099. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- 7.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Fraser I. Volume thresholds and hospital characteristics in the United States. Health Aff (Millwood) 2003;22(2):167–177. doi: 10.1377/hlthaff.22.2.167. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Dimick JB, Staiger DO. Operative mortality and procedure volume as predictors of subsequent hospital performance. Ann Surg. 2006;243(3):411–417. doi: 10.1097/01.sla.0000201800.45264.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 12.ACC/AHA 2004 Guideline Update for Coronary Artery Bypass Graft Surgery. Circulation. 2004;110(14):e340–e437. [PubMed] [Google Scholar]
- 13.Shahian DM, Edwards FH, Ferraris VA, et al. Quality measurement in adult cardiac surgery: part 1--Conceptual framework and measure selection. Ann Thorac Surg. 2007;83(4 Suppl):S3–S12. doi: 10.1016/j.athoracsur.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Glance LG, Dick AW, Osler TM, Mukamel DB. Accuracy of hospital report cards based on administrative data. Health Serv Res. 2006;41(4 Pt 1):1413–1437. doi: 10.1111/j.1475-6773.2006.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glance LG, Dick AW, Osler TM, Mukamel DB. Does date stamping ICD-9-CM codes increase the value of clinical information in administrative data? Health Serv Res. 2006;41(1):231–251. doi: 10.1111/j.1475-6773.2005.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iezzoni LI. Finally present on admission but needs attention. Med Care. 2007;45(4):280–282. doi: 10.1097/01.mlr.0000259078.54902.fe. [DOI] [PubMed] [Google Scholar]
- 17.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295(10):1168–1170. doi: 10.1001/jama.295.10.1168. [DOI] [PubMed] [Google Scholar]
- 18.Wen HC, Tang CH, Lin HC, Tsai CS, Chen CS, Li CY. Association between surgeon and hospital volume in coronary artery bypass graft surgery outcomes: a population-based study. Ann Thorac Surg. 2006;81(3):835–842. doi: 10.1016/j.athoracsur.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Ho V, Heslin MJ, Yun H, Howard L. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13(6):851–858. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Carey JS, Danielsen B, Gold JP, Rossiter SJ. Procedure rates and outcomes of coronary revascularization procedures in California and New York. J Thorac Cardiovasc Surg. 2005;129(6):1276–1282. doi: 10.1016/j.jtcvs.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer JD. High-risk surgery--follow the crowd. JAMA. 2000;283(9):1191–1193. doi: 10.1001/jama.283.9.1191. [DOI] [PubMed] [Google Scholar]
- 22.Khuri SF, Henderson WG. The case against volume as a measure of quality of surgical care. World J Surg. 2005;29(10):1222–1229. doi: 10.1007/s00268-005-7987-6. [DOI] [PubMed] [Google Scholar]
- 23.Ward MM, Jaana M, Wakefield DS, et al. What would be the effect of referral to high-volume hospitals in a largely rural state? J Rural Health. 2004;20(4):344–354. doi: 10.1111/j.1748-0361.2004.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 24.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291(2):195–201. doi: 10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 25.Christian CK, Gustafson ML, Betensky RA, Daley J, Zinner MJ. The Leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg. 2003;238(4):447–455. doi: 10.1097/01.sla.0000089850.27592.eb. discussion 455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciardi R, Virnig BA, Ogilvie JW, Jr, Dahlberg PS, Selker HP, Baxter NN. Volume-outcome relationship for coronary artery bypass grafting in an era of decreasing volume. Arch Surg. 2008;143(4):338–344. doi: 10.1001/archsurg.143.4.338. discussion 344. [DOI] [PubMed] [Google Scholar]
- 27.Marcin JP, Li Z, Kravitz RL, Dai JJ, Rocke DM, Romano PS. The CABG surgery volume-outcome relationship: temporal trends and selection effects in California, 1998–2004. Health Serv Res. 2008;43(1 Pt 1):174–192. doi: 10.1111/j.1475-6773.2007.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin HC, Xirasagar S, Tsao NW, Hwang YT, Kuo NW, Lee HC. Volume-outcome relationships in coronary artery bypass graft surgery patients: 5-year major cardiovascular event outcomes. J Thorac Cardiovasc Surg. 2008;135(4):923–930. doi: 10.1016/j.jtcvs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Bradley EH, Herrin J, Elbel B, et al. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short-term mortality. JAMA. 2006;296(1):72–78. doi: 10.1001/jama.296.1.72. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240(4):586–593. doi: 10.1097/01.sla.0000140752.74893.24. discussion 593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702. doi: 10.1001/jama.296.22.2694. [DOI] [PubMed] [Google Scholar]
- 32.Casale AS, Paulus RA, Selna MJ, et al. "ProvenCareSM": a provider-driven pay-for-performance program for acute episodic cardiac surgical care. Ann Surg. 2007;246(4):613–621. doi: 10.1097/SLA.0b013e318155a996. discussion 621-623. [DOI] [PubMed] [Google Scholar]
- 33.Shahian DM, Normand SL. Low-volume coronary artery bypass surgery: measuring and optimizing performance. J Thorac Cardiovasc Surg. 2008;135(6):1202–1209. doi: 10.1016/j.jtcvs.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Lindenauer P, Remus D, Roman S, et al. Public Reporting and Pay for Performance in Hospital Quality Improvement. 2007 Jan 26; doi: 10.1056/NEJMsa064964. Vol. www.nejm.org. [DOI] [PubMed] [Google Scholar]