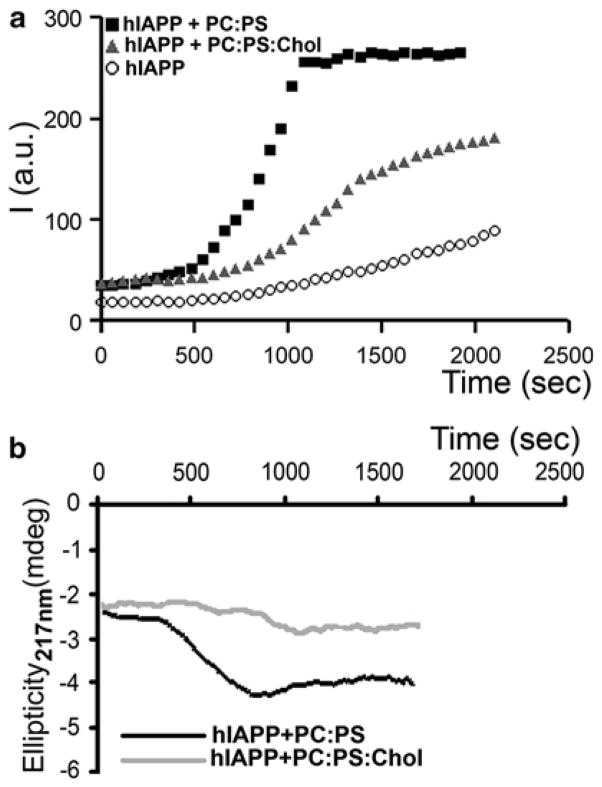
Fig. 4.3.
Membrane cholesterol and anionic phospholipids oppositely regulate amylin aggregation and misfolding in solution. (a) Thioflavin-T fluorescent assay reveals slow aggregation of 10 μM human amylin in solution (circles). Presence of 100 μM anionic liposomes (PC:PS, 2.8:1.2 mol:mol, squares) in incubating solution (PBS, 1 % HFIP) accelerates amylin aggregation, the effect of which was reversed by inclusion of cholesterol in the lipid vesicles (PC:PS:Chol, 2.3:1:0.8 mol:mol:mol, triangles). (b) Dynamics of amylin secondary structural transitions in solution are regulated by membranes. CD spectras of human amylin (10 μM) incubated with 100 μM PC:PS liposomes (2.8:1.2 mol:mol, black trace) or PC:PS:Chol liposomes (2.3:1:0.8 mol:mol:mol, gray trace) were continuously acquired at 220 nm to monitor appearance of β-sheet conformation. Note that the onset of amylin aggregation and the transition from random coil to β-sheet coincide (hA+PC:PS, Fig. 4.3a, b). Inclusion of cholesterol prolonged amylin’s transition to β-sheets evoked by anionic liposomes (Fig. 4.3b), ultimately reducing kinetics and the extent of amylin aggregation (Fig. 4.3a)