Fig. 4.5.
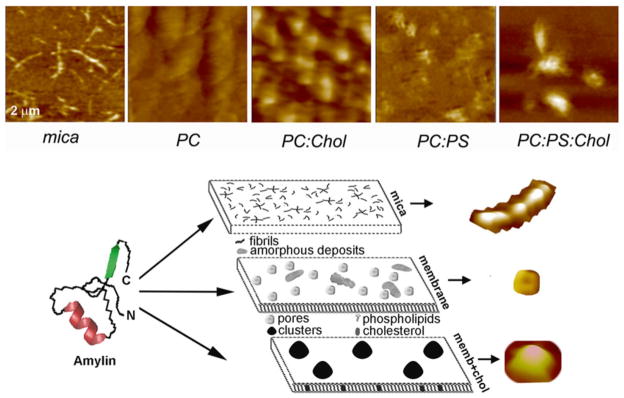
AFM analysis of membrane-directed amylin self-assembly. High-resolution 2D AFM micrographs (top panel) reveal distinct patterns of amylin aggregation and deposition on different surfaces. Note the clustering of amylin aggregates on cholesterol-containing membranes, PC:Chol (3.2:0.8 mol:mol) and PC:PS:Chol (2.3:1:0.8 mol:mol:mol) and their homogenous distribution/aggregation on cholesterol-free membranes, PC and PC:PS (2.8:1.2 mol:mol). In contrast to mica, no fibrils were detected on either membranes. Micrographs are 2 × 2 μm. Bottom panel: Proposed pathway of amylin polymerization and accumulation on different surfaces. The form and amount of amylin deposits correlate with the physicochemical properties of the supporting surface. On stiffpolar mica surface, amylin monomers (left) associate into spherical oligomers that align and elongate overtime to produce mature fibrils that randomly distribute across the surface (mica). On soft planar membranes, amylin self-assembles into globular highly symmetrical supramolecular structures featuring a central pore. Unstructured amorphous amylin aggregates are also formed on this surface (membrane). Incorporation of cholesterol into planar membranes redirects amylin surface deposition by stimulating formation of larger, but fewer amylin clusters (memb+chol). Consequently, the membrane surface area free of amylin deposits increases significantly, which diminishes amylin accumulation. Three major polymorphic forms, a fibril, a pore and a single cluster formed during amylin polymerization on different surfaces, are presented top to bottom (bottom panel)
