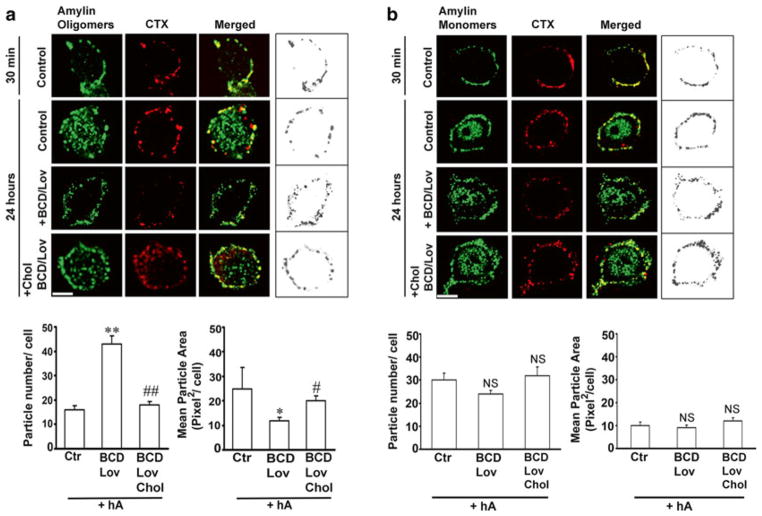
Fig. 4.6.
Binding and clustering of amylin oligomers into microdomains on the cell PM requires cholesterol. (a) Confocal microscopy analysis of amylin oligomer and cholera toxin (CTX) distribution on the cell PM. Characteristic binding profiles of amylin oligomers on the cell PM for each treatment (within boxes, right panel) are rendered in gray tones for easier particle comparisons, which are presented side by side with the original fluorescence images (left panels). Note the time-dependent increase in the number of internalized amylin oligomers (control, 30 min vs. 24 h, left panel), which prevents accumulation of amylin oligomers on the cell PM (control, 30 min vs. 24 h, right box). Single particle analysis demonstrates a threefold increase in the number of amylin oligomer clusters, or puncta, on the PM (particle no.), and their dispersion across the PM in cholesterol-depleted cells (mean particle area). Significance established at *p < 0.05, **P < 0.01 control vs. BCD/Lov, and #p < 0.05, ##p < 0.01 BCD/Lov vs. BCD/Lov/Chol. (b) Amylin monomer internalization is not blocked by cholesterol depletion. Confocal microscopy demonstrates internalization of amylin monomers both in controls (hA) and cholesterol-depleted cells (hA + BCD/Lov). No significant (NS) change in PM-binding pattern of amylin monomers (right boxes) was noticed upon cholesterol depletion. PM cholesterol does not modulate amylin deposition on the PM. The number of amylin puncta on PM (particle no.) and their area (mean particle area) did not change significantly upon depletion of PM cholesterol by BCD/Lov. Bars are 5 μm