Fig. 4.7.
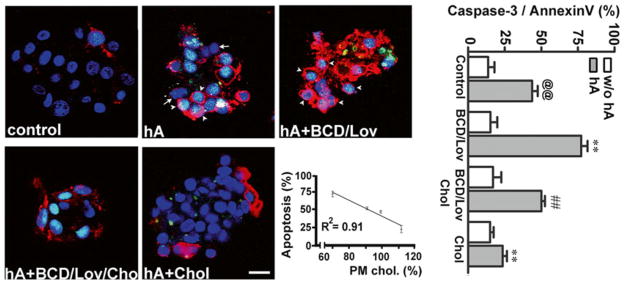
PM cholesterol prevents toxicity of soluble amylin oligomers in cultured human islet cells. Confocal microscopy analysis of phosphatidylserine (PS) externalization and caspase-3 proteolytic activation by amylin in cells with normal, depleted and enriched cholesterol levels (micrographs, left panel). Arrows depict non-apoptotic nuclei (blue) in viable cells, whereas arrowheads depict fluorogenic caspase-3 substrate found in the nuclei of apoptotic cells, giving these nuclei a green/blue appearance. The majority of PS-positive cells (red) show shrinkage and nuclear condensation (arrowheads) indicative of apoptosis (hA and hA+BCD/Lov). Bar is 10 μm. Linear regression analysis shows an inverse relationship between amylin-induced cell death and PM cholesterol levels. The extent of cell death evoked by amylin was plotted as a function of variable PM cholesterol levels in controls and treatments (apoptosis vs. cholesterol, graph). Quantitative analysis of amylin-induced apoptosis in human islets (caspase-3/annexin, graph) reveals a significant increase in amylin toxicity in cholesterol-depleted cells as compared to controls (@@p < 0.01 hA vs. control, **p < 0.01 hA vs. treatments, right panel). Replenishment of PM cholesterol levels significantly decreased amylin toxicity (##p < 0.01 hA+BCD/lov vs. hA+BCD/Lov/Chol)
