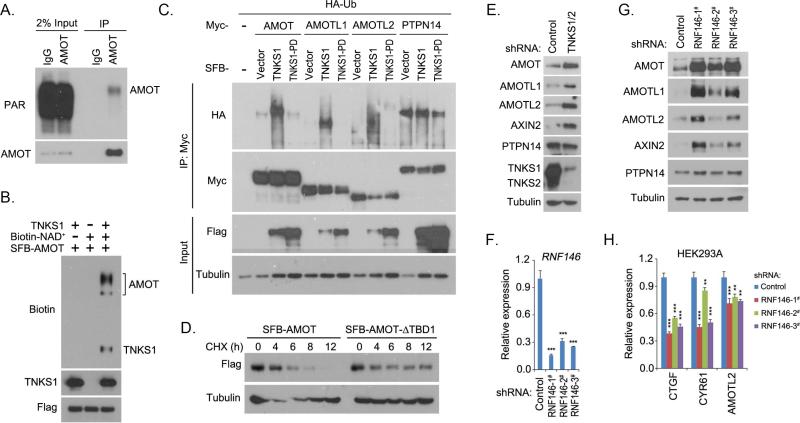
Figure 3. Tankyrase-RNF146 axis promoted the degradation of AMOT proteins.
(A) AMOT protein was ribosylated in vivo. Endogenous immunoprecipitation (IP) was performed using extract prepared from HEK293T cells containing ADP-HPD (5 μM); normal rabbit IgG was taken as control. PAR, poly-ADP ribosylation polymer antibody. (B) Tankyrase 1 (TNKS1) promoted ribosylation of AMOT protein in vitro. In vitro ribosylation assay was performed and the ribosylated proteins were indicated by the incorporation of biotinylated NAD+ and detected by anti-biotin antibody. (C) Tankyrase 1 (TNKS1) induced the ubiquitination of AMOT proteins. HA-tagged ubiquitin (Ub) was co-expressed with indicated proteins in HEK293T cells for 24 h. Cells were treated with proteasome inhibitor MG132 (10 μM) for 6 h and subjected to immunoprecipitation (IP) assay. TNKS1-PD is the inactive mutant for TNKS1. (D) Tankyrase-binding domain (TBD) deletion mutation stabilized the AMOT protein. AMOT or its TBD-deleted mutant (AMOT-ΔTBD1) was expressed in HEK293T cells for 24 h. Cells were treated with cycloheximide (CHX; 100 μg/mL) and collected at different time points for Western blotting. (E) Loss of tankyrase 1/2 stabilized AMOT proteins. Indicated proteins were detected in control and tankyrase 1/2 knockdown (shRNA-transduced) HEK293A cells by Western blotting. (F-G) Loss of RNF146 stabilized AMOT proteins. RNF146 knockdown efficiency was shown by quantitative PCR (F). Indicated proteins were detected in control and three groups of RNF146 shRNA-transduced HEK293A cells by Western blotting (G). (H) Loss of RNF146 suppressed the transcripts of YAP target genes. The transcripts were detected by quantitative PCR in control and three groups of RNF146 shRNA-transduced HEK293A cells. For panels F and H, ** p<0.01 and *** p<0.001.