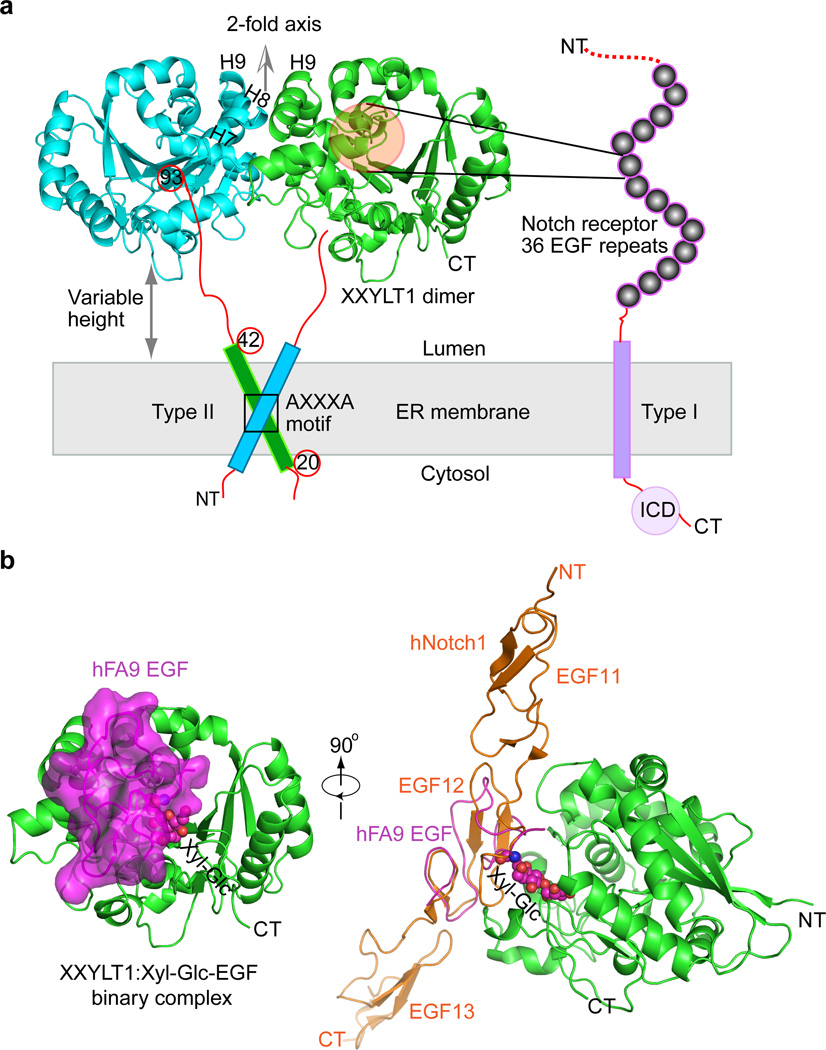
Figure 1. The mouse XXYLT1 is a dimer and has a GT-A fold with its active site facing sideways to facilitate lateral modification of Notch.
(a) The overall structure of type II membrane protein XXYLT1, with its truncated amino terminal transmembrane domain shown as a cylinder. The non-crystallographic two-fold axis is oriented upward, perpendicular to the sketched ER membrane in gray. The type I membrane protein Notch receptor is sketched with its 36 EGF repeats as spheres. NT, N-terminus; CT, C-terminus; ICD, intracellular domain; H7, Helix 7; H8, Helix 8; H9, Helix 9. (b) Left, overall structure of XXYLT1 in complex with hFA9 Xyl-Glc-EGF; right, superposition of the crystal structure of human Notch1 EGF11–13 (PDB ID 2VJ3) with the acceptor EGF, showing that the enzyme interacts laterally with the protein substrates. XXYLT1 is in green cartoon and in the same orientation as the green apo structure in (a); Xyl-Glc-EGF is in magenta cartoon with a semi-transparent surface view, and the covalently linked disaccharide is in spheres. The human Notch1 EGF11–13 is shown in orange cartoon.