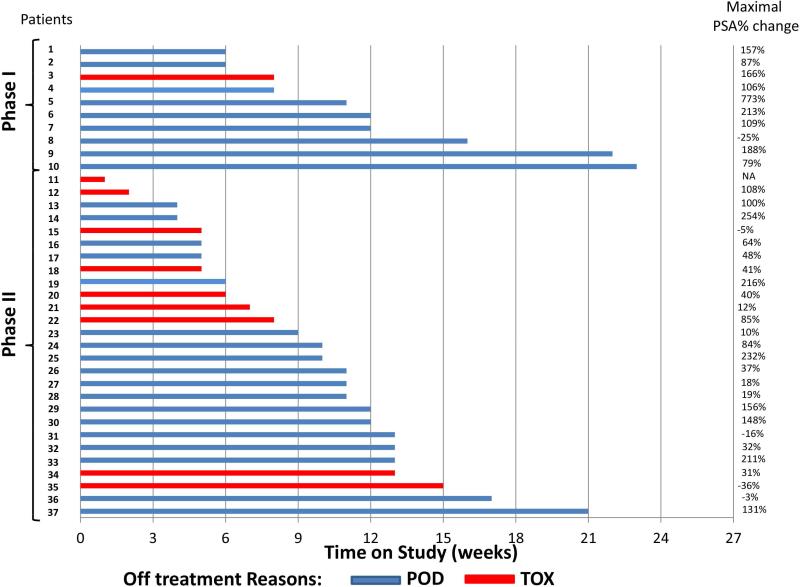
Figure 2. Time on study, maximal percent PSA change from baseline during study, and reason for discontinuation in prostate cancer patients (n=37).
PSA: prostate-specific antigen, POD: progression of disease; TOX: toxicity; NA: not available. Thirty-seven patients with castration-resistant prostate cancer were treated on study (10 phase I and 27 phase II). Median time on study was 12 weeks in phase I and 11 weeks in phase II. The majority of patients (5 phase I, 18 phase II) discontinued treatment prior to the planned 12-week evaluation due to disease progression determined by imaging and/or rise in PSA (15 patients: 4 phase I, 11 phase II), or grade ≥ 2 toxicity (8 patients: 1 phase I, 7 phase II). We examined the greatest percent PSA change from baseline for each patient at any time during the study: this constituted a PSA rise for 31 patients (ranging from 10% to 773%), a PSA decline for 5 patients (ranging from −3% to −36%), and maximal percent change could not be calculated for one patient who discontinued after only 1 week on study. Of the 5 patients whose maximal PSA change was a decline, median time on study was 15 weeks.