Abstract
Background
Activatable near-infrared fluorescent (NIRF) probes have been used for ex vivo and in vivo detection of intestinal tumors in animal models. We hypothesized that NIRF probes activatable by cathepsins or MMPs will detect and quantify dextran sulphate sodium (DSS) induced acute colonic inflammation in wild type (WT) mice or chronic colitis in IL-10 null mice ex vivo or in vivo.
Methods
WT mice given DSS, water controls and IL-10 null mice with chronic colitis were administered probes by retro-orbital injection. FMT2500 LX system imaged fresh and fixed intestine ex vivo and mice in vivo. Inflammation detected by probes was verified by histology and colitis scoring. NIRF signal intensity was quantified using 2D region of interest (ROI) ex vivo or 3D ROI-analysis in vivo.
Results
Ex vivo, seven probes tested yielded significant higher NIRF signals in colon of DSS treated mice versus controls. A subset of probes was tested in IL-10 null mice and yielded strong ex vivo signals. Ex vivo fluorescence signal with 680 series probes was preserved after formalin fixation. In DSS and IL-10 null models, ex vivo NIRF signal strongly and significantly correlated with colitis scores. In vivo, ProSense680, CatK680FAST and MMPsense680 yielded significantly higher NIRF signals in DSS treated mice than controls but background was high in controls.
Conclusion
Both cathepsin or MMP-activated NIRF-probes can detect and quantify colonic inflammation ex vivo. ProSense680 yielded the strongest signals in DSS colitis ex vivo and in vivo, but background remains a problem for in vivo quantification of colitis.
Keywords: molecular imaging, near-infrared, colonic inflammation
Introduction
Inflammatory disorders of the small bowel and colon span a clinical spectrum from acute mucosal injury and transient inflammation to chronic inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease (1). Clinical presentation combined with conventional endoscopy/ileocolonoscopy and histopathology represent the mainstays of diagnosis and detection of intestinal inflammatory diseases (1, 2). Additional imaging methods are desirable to improve detection and diagnosis of inflammation in the small or large intestines (3–5).
There is expanding interest in the application of optical imaging with near infrared fluorescent (NIRF) probes to detect inflammation. Imaging in the near infrared (NIR) region has potential advantages for deep tissue imaging, and can minimize light scattering and problems associated with non-specific tissue autofluorescence at low wavelengths (6–8). NIRF probes activated by cathepsins and matrix metalloproteinases (MMPs) have been used for in vivo imaging of inflammation in several organs in rodent models although emphasis to date has been on superficial organs rather than more deeply situated organs such as colon (6).
Cathepsins and MMPs are known to be up-regulated in human IBD and preclinical models such as acute intestinal injury and inflammation induced by dextran sulfate sodium (DSS) or chronic colitis in the interleukin-10 (IL-10) null mouse (9–14). A majority of studies in IBD or animal models have evaluated cathepsins and MMPs at the level of expression of mRNA or protein (9, 15–18) and multiple isoforms of these enzymes are expressed in normal or inflamed intestine (9, 16, 18). The ability to detect and quantify activated cathepsins or MMPs ex vivo or in vivo may provide advantages for detection and localization of intestinal inflammation and evaluation of disease severity in high throughput preclinical studies. In the present study, a series of commercially available near infrared fluorescence (NIRF) probes activated by cathepsins or MMPs were compared for ex vivo or in vivo detection of inflammation in the colon of mice with DSS induced acute colonic injury and inflammation. A subset of probes was also tested in the IL-10 null model of chronic colitis. The probes are summarized in Table 1 and comprise polymer-based substrates that are internally quenched by the near infrared fluorophores loaded onto the backbone structure, but are activated to emit NIRF when cleaved by cathepsins or MMPs (19). ProSense and MMPSense probes have been successfully used to detect inflammation in preclinical models of asthma (20) and atherosclerosis (21) and have been widely used to detect tumors, including intestinal tumors (22, 23). However, their utility for detection and quantification of colonic inflammation has not been quantitatively evaluated or compared. A probe with high specificity for cathepsin K was also tested in this study because immunohistochemical studies suggest that cathepsin K may have diagnostic potential in Crohn’s disease (17). Our specific goals in this study were to define if one or more probes reliably detects and quantifies experimental inflammation ex vivo or in vivo and to compare cathepsins and MMP-based probes activated at 680 or 750 wavelengths as well as new FAST probes which permit more rapid imaging.
Table 1.
Activatable NIRF probes used to detect intestinal inflammation
| Probes | Target | Dosage/mouse | Injection before imaging |
|---|---|---|---|
| PS 680 | Cathepsins | 2 nmols/150ul | 24 hours |
| PS 750 | Cathepsins | 2 nmols/150ul | 24 hours |
| MMPS 680 | MMPs | 2 nmols/150ul | 24 hours |
| Cat B 680 FAST | Cathepsin B | 2 nmols/100ul | 6 hours |
| Cat K 680 FAST | Cathepsin K | 2 nmols/100ul | 6 hours |
| PS 750 FAST | Cathepsins | 4 nmols/100ul | 6 hours |
| MMPS 750 FAST | MMPs | 2 nmols/100ul | 6 hours |
Abbreviation: PS: ProSense; MMPS: MMPSense
Several fluorescence-based imaging techniques are available. Macroscopic fluorescence reflectance imaging (FRI) is a useful technique when probing superficial structures (<5mm deep). In this study, FRI was tested for ability to detect and quantify inflammation ex vivo in the colon of DSS and IL-10 null models. Ability to detect inflammation based on activated probe was evaluated in both freshly dissected and fixed tissues. The ability to detect and quantify inflammation in fixed tissue offers potential advantages for studies in large numbers of animals. Fluorescence molecular tomography (FMT) potentially permits three-dimensional (3D) and deep localization and quantification of fluorescent probes in vivo. FMT was used for in vivo live imaging in the DSS model to assess whether FMT could reliably detect colonic inflammation.
Signals obtained with the most promising probes were tested for correlations with histologic scoring of disease severity. Our study demonstrates that both cathepsin and MMP-activated probes detect and quantify colonic inflammation ex vivo with 680 activated probes providing stronger signals than 750 or FAST probes. Probes show promise for in vivo monitoring but improved background and ROI imaging specifically targeted to the colon is desirable.
Materials and Methods
Mouse models
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill (Protocol number: 13-162).
WT C57BL6 mice were used for DSS induced acute mucosal injury and inflammation studies. Adult (8–12 weeks) mice were given 3% DSS (TdB consultancy AB, Sweden) in drinking water for 5 days, then switched to water for the following 4 days. Mice were studied four days after cessation of DSS since prior studies demonstrated that acute DSS-induced inflammation is maximal at 3–4 days after DSS (24). Mice given free access to water served as H2O controls. Control imaging experiments revealed autofluroescence of chow diet and fecal pellets from chow fed mice at NIR wavelengths which could be minimized by feeding a liquid diet (see supplemental figure 1 and details of control imaging experiments below). Therefore, both DSS-treated mice and water controls were given liquid diet (Nestle Nutrition, Minnetonka, MN) (Nutren 1.0 Fiber : dH2O=1:1) instead of normal, solid chow for 4 days before in vivo imaging to clear the bowel of solid fecal matter and minimize autofluorescence. Liquid diet was prepared fresh daily and given ad libitum in mouse feeding bottles (Cat. 9019 and 9015, Bio-serv, Frenchtown, NJ). The mice were housed in cages with wire mesh bottoms to prevent coprophagia. Water was provided ad lib throughout the period of liquid diet feeding.
Germ-free IL-10 null and WT mice on 129 SvEv background were obtained from the Gnotobiotic rodent models core facility at UNC-CH and were transferred to a specific pathogen free (SPF) facility for 4 months before imaging experiments to allow spontaneous chronic colitis to develop in the genetically susceptible IL-10 null mice. As with DSS, IL-10 null and WT mice were given liquid diet prior to imaging.
Molecular imaging methodology
The commercially available enzyme activatable NIRF imaging probes (PerkinElmer) listed in Table 1. were given via retro orbital injection. All probes are optically silent until cleaved and activated by proteases. The fluorophore on 680 series probes is excited by light at 680±10 nm and emits light at 700±10nm. The fluorophore on 750 series probes is excited at 750nm and emits at 770nm. ProSense 680, ProSense 750 and MMPSense 680 were given to animals 24 hours before imaging according to manufacture instructions (PerkinElmer). FAST (Fluorescent Activatable Sensor Technology) imaging agents designed to permit earlier imaging were given 6 hours before imaging as per manufacture instructions (PerkinElmer). Each probe was diluted in 1X phosphate buffered saline (1X PBS) before injection and at doses recommended by the manufacturer. Published reports indicate up-regulation of both cathepsins and MMPs in damaged and acutely inflamed colon of DSS treated mice (12, 13). The peptide substrate of ProSense 680 is activated by proteases such as cathepsin B, L, S and plasmin with reportedly highest specificity for cathepsin B (21). MMPSense substrate is cleaved by key MMPs including MMP-2, -3, -9 and -13 with reportedly highest specificity for MMP-9 (according to manufacturer).
Control ex vivo imaging experiments
Prior to large scale experiments, with multiple activatable NIRF probes in DSS or IL-10 null mice, control experiments were performed to ensure minimal background autofluorescence. Imaging of fecal pellets from chow fed WT mice reveal autofluroscence (Supplemental Figure 1A). Imaging of chow diet alone also revealed autofluroescence whereas a liquid diet had minimal autofluorescence (Supplemental Figure 1B). Imaging of fecal pellets from water controls or DSS treated mice given no probe injection revealed higher autofluorescence of fecal pellets from water controls versus DSS treated mice (Supplemental Figure 1C). This autofluorescence and difference between water controls was eliminated by liquid diet feeding for 4 days before imaging (Supplemental Figure 1D). Together, these pilot studies led us to use liquid diet feeding prior to probe injection and imaging as a standard protocol. Ex vivo imaging of small intestine and colon from water controls or DSS treated mice given the liquid diet protocol but no probe injection, then subject to FRI imaging at the 680 or 750 wavelength demonstrated minimal background and no difference between water controls and DSS treated mice (Supplemental Figures 1E and 1F). Together the findings from these experiments suggested that pre-imaging liquid diet and inclusion of no probe controls, and disease-free controls given probe represent important controls to minimize background autofluorescence and validate specificity of signals in diseased animals. Even with liquid diet, we note that is essential to carefully flush luminal contents from colon because remnant luminal contents in folds of the proximal colon can represent a source of non-specific fluorescence.
Ex vivo tissue FRI imaging in colitis models and controls
Animals were euthanized by intraperitoneal (i.p.) injection of Nembutal (100mg/kg body weight/per animal) 24 hours or 6 hours (FAST probes) after probe injection. Ileum and colon were dissected and gently flushed with ice cold 1X PBS and imaged ex vivo by FRI (fluorescence reflectance imaging) (FMT 2500™ LX System, PerkinElmer). Light source energy level, exposure time and the distance from specimen to camera are kept the same across animal in each experiment. To quantify fluorescence signals, region of interests (ROIs) were set manually to cover entire distal colon and the entire distal half of the proximal colon because these are regions typically involved in DSS-induced disease. The size of ROIs across animals is similar and examples of ROIs are shown in Figure 1A. The goal was to calculate the total counts due to activated probe in the entire ROI (distal colon and distal half of proximal colon) for each animal. The unit (counts/energy) in ex vivo FRI represents the counts per pixel, normalized to laser energy level. The total counts reflect the total amount of fluorescence in the entire ROI for each animal, treatment group or probe. During FRI imaging with each probe, the imaging system automatically adjusts the scale to encompass the minimum-maximum of the signal for optimal visualization with that particular probe. A higher scale reflects a stronger signal. Diseased and control animals (DSS and water controls or IL-10 null and WT) were always imaged in the same experiment. With each probe, mean of total count per ROI in controls was subtracted from the total counts per ROI for each diseased animal or water control as a measure of specific probe activation.
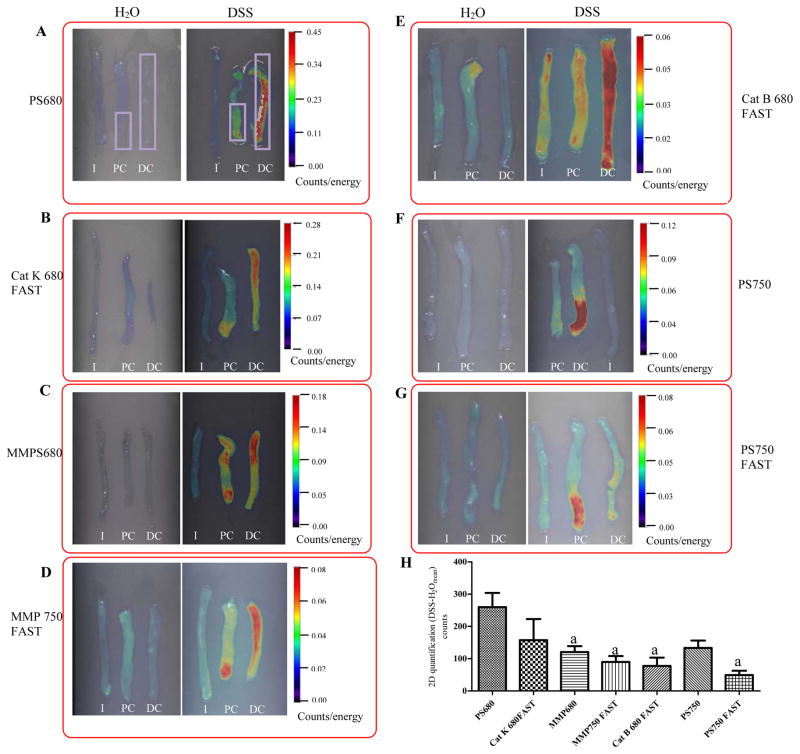
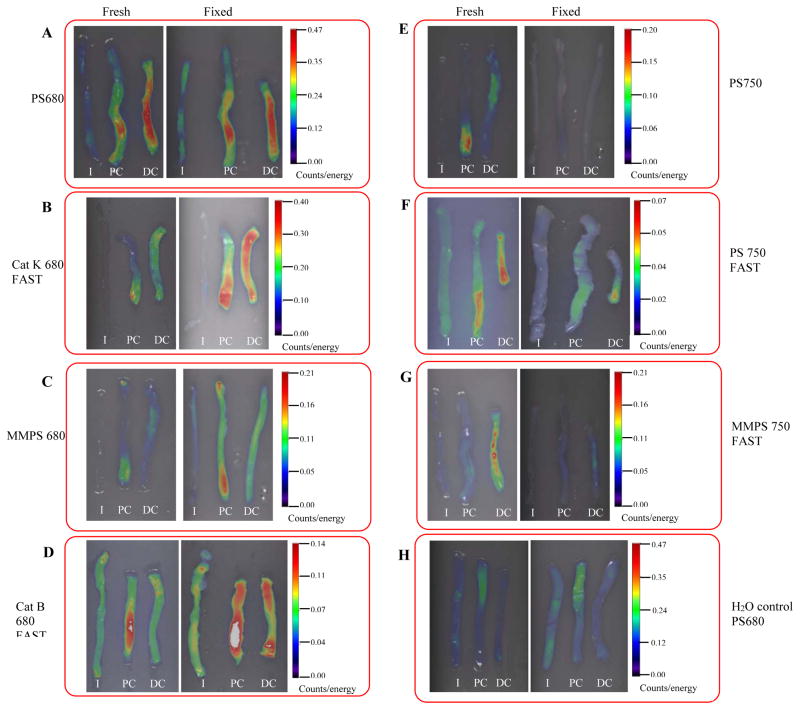
Figure 1. Strong and significant activation of all probes were detected in distal colon of DSS treated mice by ex vivo FRI imaging.
Representative 2D FRI images of fresh intestine from DSS-treated mice versus water controls are shown in Figure 1A through Figure 1G. Rectangle ROIs (in purple color) were placed on distal colon and distal half of proximal colon to quantify fluorescence signal (counts). (H) 2D Quantification of fluorescence signals in colon from DSS-treated animals (n=4–9 animal/group). Data are mean±SEM of total counts per colon ROI in each DSS treated animal injected with each of the indicated probes after subtraction of the mean value in water controls.
I:ileum; PC: Proximal colon; DC: Distal colon
a: p<0.05 vs. PS 680 (ProSense 680)
We note that the calculation of total counts per ROI was used in this study instead of ‘target to background ratio’ which is commonly used in other applications as a measure of difference in signal intensity between tumor versus normal tissue (20, 21, 25). This is because a major goal was to test if the NIRF signal in the entire colon ROI can estimate disease severity. A second goal was to test which of the different probes tested yielded significantly higher NIRF signal in colitis models than the background observed in non-diseased controls.
Intestinal tissue fixation and re-imaging
Immediately after ex vivo imaging of freshly dissected small intestine or colon, tissues were fixed in 10% zinc formalin for 24 hours, then kept in 1 X PBS at 4°C before re-imaging to test if fluorescent signal was preserved. Tissue was kept in the dark during both fixation and storage. Our pilot studies (Supplemental Figure 2) indicated that 10% zinc formalin fixation preserved activated ProSense 680 probe better than 4% paraformaldehyde (PFA) fixation. The impact of formalin fixation was therefore evaluated on the signal obtained with each of the other probes tested.
Co-localization of ProSense680 activation with Cathepsin B immunofluorescence staining
Cathepsin B was stained by immunofluorescence on formalin fixed, paraffin embedded sections of colon from DSS treated mice. Sections were deparaffinized in xylene, rehydrated in graded ethanol solutions, pretreated with 0.05M Tris Triton (TT) epitope retrieval solution at room temperature (RT) for 30 minutes and blocked in 5% normal goat serum (NGS) diluted in TT buffer for 1 hr. Cathepsin B antibody (Abcam, Cat# ab33538) was diluted in 5% NGS/TT buffer and sections were incubated with primary antibody overnight at 4°C in a humidity chamber. After washing 3 times in 0.05M Tris buffer, the sections were incubated with secondary antibodies for 1 hour at room temperature. Following three washes with 0.05M Tris, coverslips were mounted in gel-mount with DAPI (Electron Microscopy Sciences, Hatfield, PA). Activated ProSense 680 probe and cathepsin B immunofluorescence were both imaged in the same sections using a Leica TCF SPE confocal microscope (Leica, Bannockburn, IL) and an excitation wavelength of 680nm and emission wavelength of 710nm.
Histological scoring and comparison with quantitative data for NIRF probe activation
The distal half of the colon of DSS-treated mice or IL-10 null mice was divided into 8–9 segments, paraffin embedded, and hematoxylin and eosin (H&E) stained sections prepared from each segment. H&E stained sections were scored for inflammation and injury according to previously published methods (24). Histological scoring on each segment was performed by three independent investigators blinded to treatment group and averaged for inflammation severity, inflammation infiltration and crypt loss. Colon from 129 SvEv IL-10 null mice was scored as previously described (26). Probe activation in each segment was quantified by ex vivo FRI imaging. Linear regression analysis compared histological scores and 2D FRI quantification data for each colon segment.
In vivo imaging
For in vivo imaging, mice were anesthetized with Nembutal (50mg/kg body weight) via i.p. injection and abdominal and torso hair was removed using Nair lotion (Ovation Pharmaceuticals Inc. Deerfield, US) to permit light transmission. Anesthetized mice were then carefully positioned into the imaging cassette and placed into the imaging chamber of the FMT 2500™ LX system. This system uses an NIR laser diode to transilluminate (passedight through) the body of the mouse and a thermoelectrically cooled CCD camera placed on the opposite side of the imaged animal detects NIRF signal. Optical filters collect both excitation and emission fluorescence datasets. The multiple emitted fluorescence datasets were normalized to the default laser excitation data (set by manufacturer) to calculate signals. The entire image acquisition sequence took approximately 3–5 min per mouse.
3D ROI analysis quantified fluorochrome in the entire abdominal area of each mouse. Images were displayed as rotatable reconstructed datasets, allowing views in transverse, sagittal and coronal planes. A threshold was applied to all animals equal to 30% of the mean signal intensity in ROIs of water control mice. Total amount of fluorochrome in the target ROI was then automatically calculated from the reconstructed images using FMT’s TrueQuant Imaging Software (Ver. 2.0.0.19) and pre-acquired calibrations for each specific probe. Data are expressed as absolute total pmol of activated fluorescent probe per animal based on comparison with fluorescence of a phantom containing known amounts of activated probe imaged with FMT 2500LX. To calculate the extent of probe activation in DSS treated mice versus water controls, the mean signal in water controls was subtracted from the signal observed in each individual DSS treated animal.
Statistical Analysis
Total NIRF signal per colon ROI was calculated for each DSS or H2O treated animal injected with each probe. The mean value in probe injected water controls run in the same experiment was subtracted from each individual value to normalize for background. Data for each treatment group and probe were then expressed as mean ± SE. Students test for significant difference in the mean signal obtained in DSS versus water controls injected with the same probe. ANOVA tested for significant differences in mean signals in DSS treated animals injected with each of the different probes. Tukey’s post hoc test was then used for comparisons between probes.
Results
Ex vivo imaging reveals strong and significant activation of all probes in distal colon of DSS treated mice
Ex vivo FRI imaging and 2D quantification were performed on dissected ileum, proximal colon and distal colon of DSS-treated mice and water controls. Ileum from DSS treated mice is a useful negative control as it is well established that DSS induces disease in colon and not small intestine, with disease most severe in distal colon (27). Representative 2D FRI images from probe injected H2O controls and DSS treated animals are shown in Figure 1. Figure 1 demonstrates that all probes yielded intense signals in distal colon of DSS-treated mice with little or no detectable signal in ileum of DSS treated mice. Signals were undetectable or very low in colon and ileum of water controls (Figure 1). Note that the pseudocolor scale for each probe is different. For each probe, the scale is auto-adjusted to minimum-maximum of the signal for optimal visualization, but with each individual probe the same scale is used for DSS and water controls. A lower maximum value for each probe reflects a weaker signal. Total counts in the distal colon and distal half of the proximal colon from 4–9 animals in each group (treatment or particular probe) were quantified to assess the absolute increase in signal in colon of DSS-treated mice vs. mean signal in water controls. Mean and SEM of signals obtained with each of the tested probes are illustrated in Figure 1H. Each probe tested yielded signals in colon ROI of DSS treated mice that were significantly above background in water controls. Of the probes tested in DSS-treated mice, ProSense 680 yielded the highest signals compared with signals obtained with other probes and ProSense 680, CatK fast and Prosense 750 yielded signals that were significantly higher than other probes tested (p<0.05) (Figure 1H).
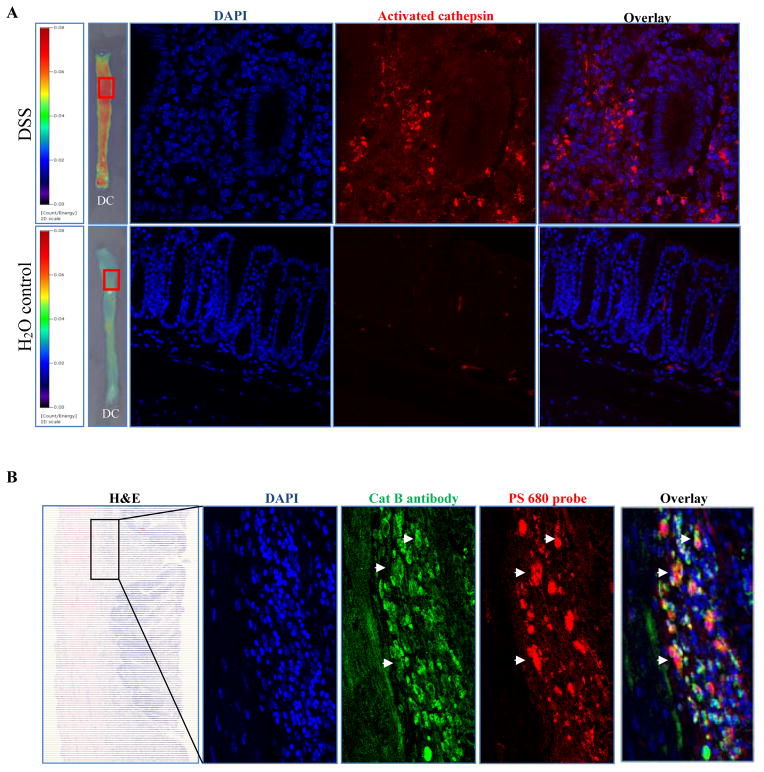
Activation of ProSense 680 in distal colon of DSS treated animals was further confirmed by confocal microscopy using excitation light at 680 nm while signal was virtually undetectable in water controls (Figure 2A). Overlap between immunofluorescence staining observed with a cathepsin B antibody and activated ProSense 680 on the same section from DSS-treated mice (Figure 2B) provided additional evidence that cathepsin B enzyme activation was validly detected by the ProSense 680 activatable probe.
Figure 2. ProSense 680 probe activation co-localized with cathepsin B antibody immunofluorescence staining.
(A) Cathepsin enzyme activation was detected only in colon from DSS treated animal, but not in colon from water control animal. (B) Co-localization of ProSense 680 probe activation and cathpsin B antibody by immunostaining. White arrows indicate the overlap of enzyme activation and cathepsin B antibody staining.
DC: Distal colon
NIRF imaging signal correlates with histological scores for colitis severity
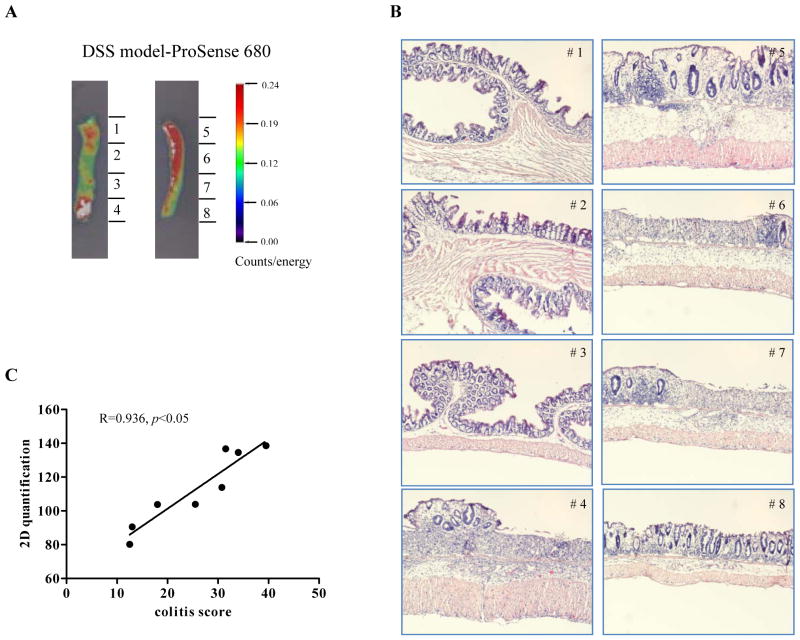
Histologic scoring of colitis was performed on H&E stained sections from different segments of colon from DSS-treated mice as illustrated in Figure 3A and 3B. Ex vivo FRI quantified NIRF signal for each of the segments (total counts). Linear regression revealed a very strong and significant correlation between ex vivo FRI signals obtained for ProSense 680 and histologic colitis scores. Regression analyses on histologic colitis scores and FRI data on colon segments from animals injected with MMP680 or Cat B680 FAST also revealed strong and highly significant correlations (MMPSense680 R=0.854, p<0.001; and CatB 680 FAST R=0.938, p<0.0001)
Figure 3. NIRF imaging signal correlates with histological scores for DSS-induced acute colonic inflammation severity.
(A) Illustrative photograph of colon segments processed for histologic colitis scoring and 2D FRI quantification of NIRF signal obtained with ProSense 680. (B) H&E-stained picture on each segment from the same animal. (C) Linear regression analysis on NIRF signal versus colitis score from each segment.
To investigate if FRI signals obtained with activated probes across individual animals treated with DSS also correlated with individual colitis scores, five animals were given MMP680 and regression analysis performed on FRI data on entire distal colon and distal half of proximal colon and histological colitis scores on tissue sections. A strong and significant correlation across individual DSS-treated mice (R=0.829; p<0.05) provides evidence that NIRF imaging validly detects differences in inflammation severity across animals.
Multi-channel imaging of activated MMP or ProSense probes in a single animal detect similar patterns of probe activation
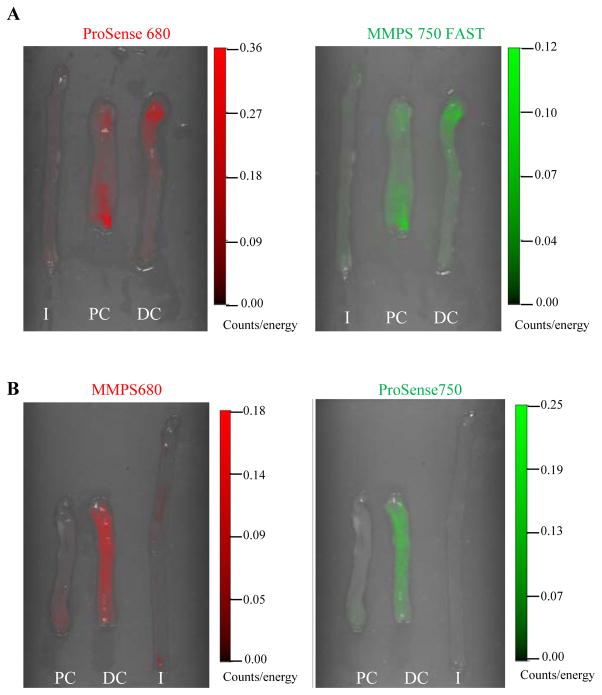
One potential advantage of fluorescence optical imaging over other imaging modalities is the ability to use multiple imaging channels to quantify activation of multiple probes in a single animal (6). To test this in models of colon inflammation, the same DSS-treated mice were injected with both cathepsin activatable and MMP activatable probes. Ex vivo images obtained by FRI at 680 and 750 excitation wavelengths in the same DSS-treated mouse injected with either ProSense 680 and MMPSense 750 FAST or MMPSense 680 + ProSense 750 are shown in Figure 4. Cathepsin and MMP probe activation detected by individual NIRF probes in the colon from the same DSS-treated animal, revealed similar location of activation (Figure 4).
Figure 4. Dual-channel imaging of activated MMPsense or ProSense probes in a single animal detects similar patterns of probe activation.
(A) Activation of ProSense 680 and MMPSense 750 FAST probes in the same DSS-treated animal was detected by ex vivo 2D dual-channel FRI imaging. (B) Activation of MMPSense 680 and ProSense 750 probes in the same DSS-treated animal was detected by ex vivo 2D dual-channel FRI imaging.
I:ileum; PC: Proximal colon; DC: Distal colon
10% zinc formalin fixation preserved fluorescence signals from 680 fluorophore conjugated probes, but not signals from 750 fluorophore conjugated probes
One potentially useful application of the activatable imaging probes is to offer a rapid and objective measure of colitis severity, especially in experiments involving large numbers of animals. In such applications, preservation of signal after fixation would offer the opportunity to archive samples over the course of large experiments and subsequently quantify signals by FRI. Initial studies in tissues from mice injected with ProSense 680 suggested that 10% zinc formalin preserved the fluorescence signal but 4% PFA did not (Supplemental Figure 2). We therefore fixed ileum and colon in 10% zinc formalin in the dark for 24 hours and then stored in 1X PBS for at least 3 days. Representative FRI images on fresh and fixed tissues are shown in Figure 5. In animals injected with 680 fluorophore conjugated probes, FRI imaging of fresh and fixed tissue demonstrated that the strong signals in inflamed colon from DSS treated animals were preserved after fixation (ProSense 680, Cat K 680 FAST, MMPSense 680 and Cat B 680 FAST) (Figure 5A–5D). With 680 series probes, signals were qualitatively stronger in fixed versus fresh tissue although the general pattern of activation was preserved (Figure 5A–D). This was not the case with 750 series probes. As shown in Figure 5E–5G, signal intensity in the colon from animals injected with 750 probes was greatly reduced or absent after fixation. The increased signal after fixation observed with 680 probes may be caused by a general background increase since FRI on colon of water controls injected with ProSense 680 probe also revealed a qualitatively stronger signal in fixed tissue compared with fresh tissue although this is still clearly much less than in DSS treated mice (Figure 5H, compare with 5A). However, this does emphasize that imaging of fixed tissue from models of inflammation requires parallel analyses of appropriate uninflamed controls to account for quantitative changes in background with fixation.
Figure 5. 10% zinc formalin fixation preserved fluorescence signals from 680 fluorophore conjugated probes, but not signals from 750 fluorophore conjugated probes.
Fresh ileum and colon from both DSS-treated and water control animals were fixed in 10% zinc formalin for overnight and kept in 1XPBS in the dark before re-imaging. Activation pattern of 680 fluorophore conjugated probes was preserved after 10% zinc formalin fixation (A–D), however, Activation of 750 fluorophore conjugated probes were not retained after fixation (E–G). (H) Activation of ProSense 680 is enhanced on fixed versus fresh tissue in intestine tissue from water control animal.
I:ileum; PC: Proximal colon; DC: Distal colon
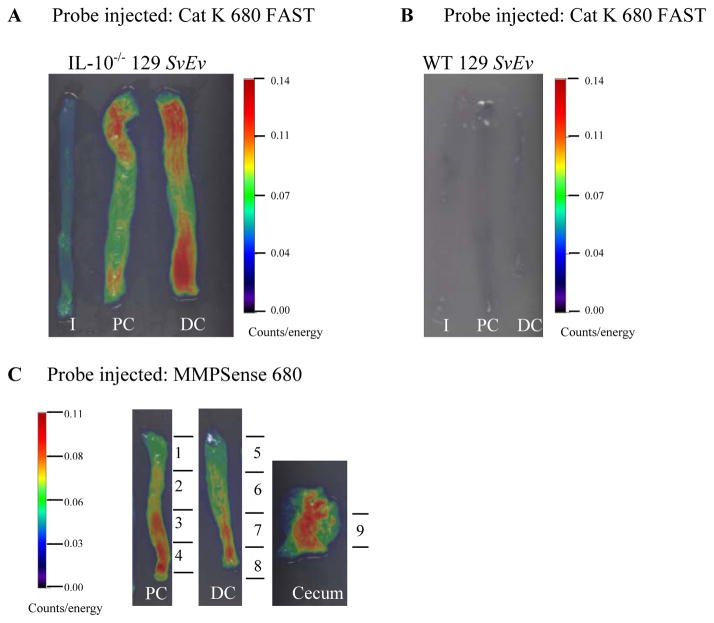
Ex vivo FRI imaging in colon tissue of 129 SvEv IL-10 null mice injected with activatable NIRF probes detects inflammation in cecum and colon
Mice on 129 SvEv background with targeted deletion of IL-10 gene spontaneously develop microbiota-driven chronic enterocolitis with massive infiltration of lymphocytes, activated macrophages, neutrophils and T cells (27, 28). To define whether NIRF probes have the ability to detect inflammation in a model of chronic inflammation with some features in common with IBD, ex vivo FRI imaging was performed on a group of 129 SvEv IL-10 null mice and WT 129 SvEv controls injected with Cat K 680 or MMPSense 680 probes. As shown in Figures 6A and 6C, both Cat K 680 FAST and MMP Sense 680 detected strong signals in proximal and distal colon and cecum (only MMP Sense 680 shown) of IL-10−/− 129 SvEv mice while signals were undetectable in ileum of IL-10−/− 129 SvEv or WT 129 SvEv controls (Figure 6A, 6B). Regression analyses on FRI signals obtained with each probe and histologic colitis scores revealed strong and significant correlations (Cat K 680 FAST, R=0.724; p<0.05; MMP Sense 680 R=0.757; p<0.05). Thus in a model of chronic colitis, activatable cathepsin-based NIRF probes detect inflammation in the regions of intestine known to be diseased in the IL-10 null model (cecum and colon but not ileum) and NIRF signal correlates with histologic disease severity.
Figure 6. Ex vivo FRI imaging in colon tissue of 129 SvEv IL-10 null mice injected with activatable NIRF probes detects inflammation in cecum and colon.
(A) Strong Cat K 680 FAST probe activation was detected in 129 SvEv IL-10 null mouse colon. (B) No fluorescence signal was detected in intestine tissue from 129 SvEv WT mouse. (C) Illustrative photograph of colon and cecum segments processed for colitis scoring and 2D quantification.
I:ileum; PC: Proximal colon; DC: Distal colon
Detection of activatable probes in vivo requires strategies to minimize background autofluorescence
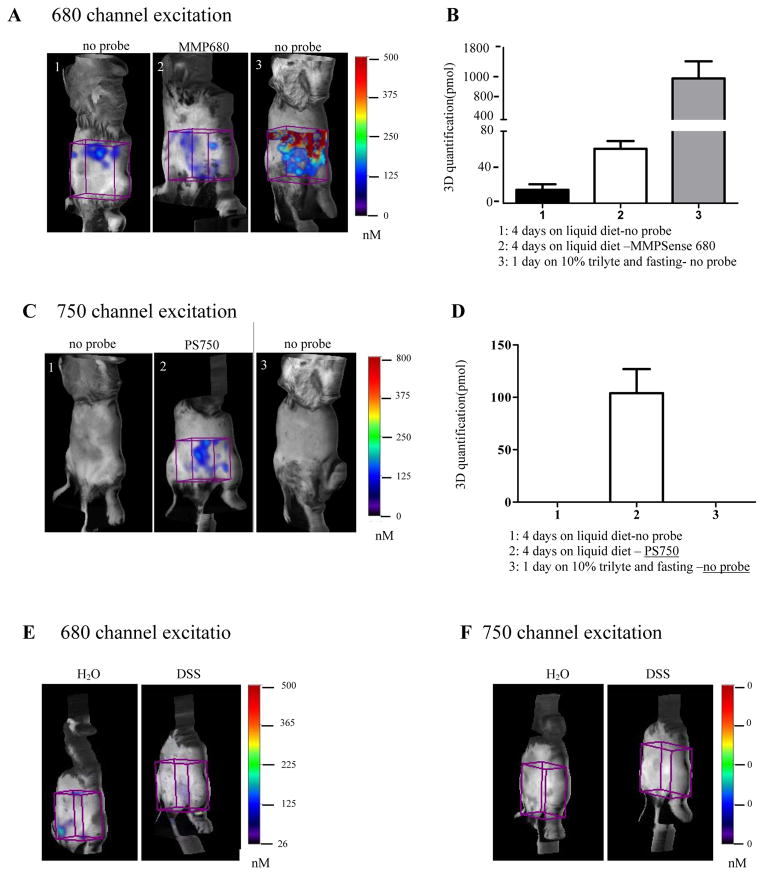
As well as testing the utility of enzyme activatable probes for ex vivo detection and quantification of injury or inflammation, it is highly desirable to identify probes and imaging modalities for in vivo monitoring of acute or chronic intestinal inflammation. A major issue for successful in vivo imaging is the elimination of potential GI tract auto-fluorescence generated mainly by fecal contents. We performed pilot in vivo studies to attempt to optimize the GI tract preparation methods before live imaging. Given our findings that liquid diet reduced ex vivo autofluorescence, a series of in vivo studies was performed on disease-free WT C57BL6 mice to test whether that liquid diet feeding could significantly reduce abdominal auto-fluorescence or background for in vivo FMT imaging. Mice were either fed with liquid diet for 4 days, or fed with 10% trilyte (PEG-3350, Cat# NDC0091-0447-23, Seymour, IN) in water and fasted for 24 hours. The latter prep was tested to model bowel preparation used in colonoscopy in humans. Probes (MMPSense 680 or ProSense 750) were given to a subset of animals fed liquid diet. 3D quantification of total fluorescence (pmol) in abdominal ROI was performed in each animal. As shown in Figure 7A and 7B when imaged at 680nm excitation, the strongest signal was observed in the animals that received trilyte and fasting but no probe injection (1199.7±33.4 pmol in ROI). Much less signal was detected in mice given 4 days of liquid diet feeding and no probe injection (14.9±6.0 pmol), although, we observed a significantly higher signal in the abdomen of animals given liquid diet and injected with probes (Figure 7B) indicating significant background fluorescence with probes even in animals without disease. Since ex vivo imaging had demonstrated minimal fluorescence in small intestine or colon of animals given probe after liquid diet in the absence of DSS treatment (Figure 1), we conclude that the abdominal signals in animals given MMPSense 680 represent non-specific background. Nonetheless these findings demonstrate that fasting overnight combining with 10% trilyte feeding cannot effectively eliminate auto-fluorescence background in GI tract for in vivo imaging using NIRF probes, and that 4 days on liquid diet reduced non-specific abdominal auto-fluorescence.
Figure 7. Liquid diet feeding significantly reduced gastrointestinal auto-fluorescence background during in vivo FMT imaging of mice.
(A) Representative 3D FMT imaging of mouse abdomen from WT, un-diseased control animals that received liquid diet minus or plus MMPSense 680 probe injection or Trylite and fasting but not probe. Images were acquired at excitation of 680 nm. Cube ROIs (in purple color) were placed on abdomen to quantify the absolute fluorescence signal (pmol). (B) Quantification of signal intensity in abdomen demonstrated lower auto-fluorescence with liquid diet feeding (no probe or probes versus Trilyte and fasting). Data are presented as mean±SEM, n=4–6/group. (C) Representative 3D FMT imaging of mouse abdomen from animals that received liquid diet minus or plus ProSense 750 probe injection or Trylite and fasting but no probe (n=4–6). Images were acquired at excitation of 750 nm. (D) Quantification of signal intensity in abdomen demonstrates that ProSense 750 probe yielded background fluorescence signal in probe injected mice but not animals minus probe. (E) 3D FMT imaging at 680nm excitation wavelength in H2O control and DSS treated animals given no probe injections reveals some background but no difference between H2O and DSS. (F) 3D FMT imaging at 750 nm excitation wavelength in H2O control and DSS treated animals given no probe injections reveals no background.
The same groups of mice were imaged at 750nm excitation wavelength. Figure 7C shows there were no detectable abdominal signals in mice that were not injected with probes, regardless of whether the mice were fed with liquid diet or not. Imaging of disease free mice after ProSense 750 probe injection revealed strong signals in abdominal area even after 4 days of liquid diet feeding (Figure 7D). Thus, both MMPSense 680 and ProSense 750 probes yielded some background fluorescence in the abdominal region of disease free mice even when mice were given liquid diet.
To investigate if inflamed bowel can cause more background fluorescence than normal bowel in live imaging, we did in vivo imaging on H2O controls and DSS treated mice given no probe (Figure 7E, 7F). Imaging at 680nm excitation revealed low fluorescence signals in water from both groups of animals and no significant higher fluorescence background was observed on DSS controls or DSS treated animals even in the absence of probe but background values did not differ between H2O controls (46.4 ± 11.7 pmol in H2O controls vs. 48.6 ± 13.6 pmol in DSS mice) (Figure 7E). The same groups of mice were imaged at 750nm excitation wavelength, and there were no detectable abdominal signals in both groups of mice (Figure 7F) indicating that background is lower with 750nm imaging.
In vivo imaging of activatable NIRF probes in water controls versus DSS treated mice
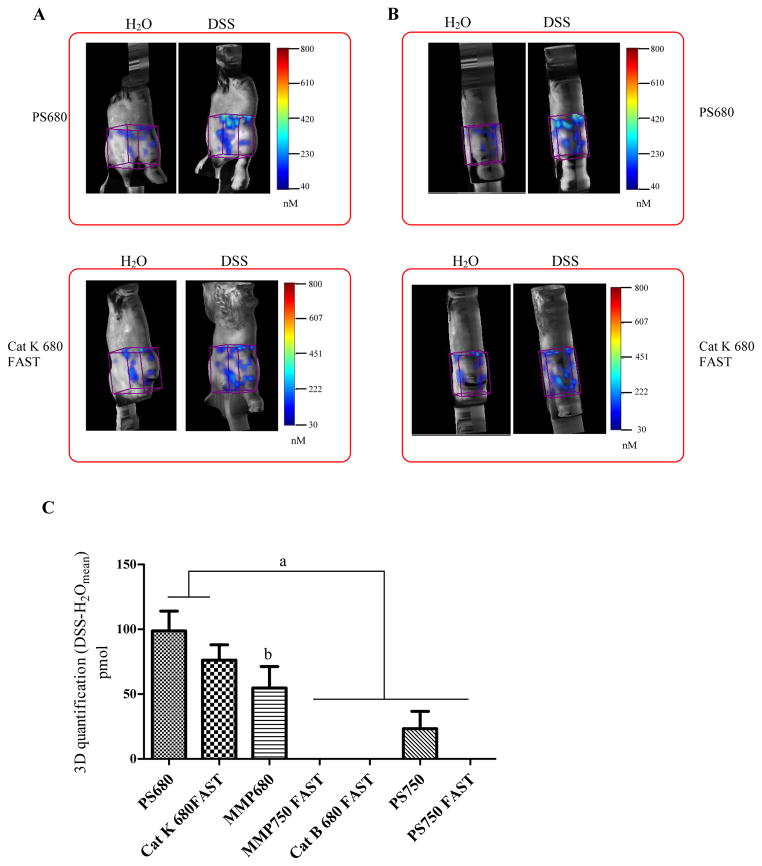
To assess whether activatable probes could detect DSS-induced colonic inflammation in vivo, FMT imaging was performed on DSS treated mice and water controls that received probe injections. All the animals were given liquid diet feeding for 4 days before imaging. Seven probes were tested for in vivo imaging, as tested ex vivo (MMPSense 680, MMPSense 750 FAST, ProSense 680, ProSense 750, ProSense 750 FAST, Cat B 680 FAST, Cat K 680 FAST). After FMT imaging, total fluorescence (pmol) was quantified in ROIs over the abdomen (see ROIs in Figure 8). Representative images of coronal (Figure 8A) and sagittal (Figure 8B) views from mice injected with ProSense 680 or Cat K 680 FAST probes are also presented in Figure 8.
Figure 8. In vivo imaging of activatable NIRF probes in DSS treated animals versus water controls.
(A) Representative 3D FMT images of abdomen on coronal plane from DSS-treated mice and water controls injected with ProSense 680 or Cat 680 FAST probes. (B) Representative 3D FMT images of abdomen on saggital plane from DSS-treated mice and water controls injected with ProSense 680 or Cat 680 FAST probes. Cube ROIs (in purple color) were placed on the abdomen to quantify the absolute fluorescence signal. (C) 3D Quantification of fluorescence signal intensity increase in abdomen from DSS-treated animals (n=4–9 animal/group). Data were presented as mean±SEM. A threshold was applied to all animals in each group equal to 30% of the mean signal intensity in ROIs of water control mice given the same probe injections.
a: p<0.05 ProSense 680 and CatK 680 fast vs. other probes; b: p<0.05 vs. MMP750 FAST vs. other probes
Mean FMT data for DSS treated mice injected with each of the seven probes tested is shown in Figure 8C. After subtracting the mean fluorescence observed in water controls, only ProSense 680, Cat K 680 FAST and MMPSense 680 yielded significantly higher signals in DSS treated mice than background observed in water controls. In vivo imaging with probes conjugated with 750 florophores and particularly FAST probes (PS750, PS750 FAST, MMPSense 750 FAST) did not yield signals significantly higher signals than the background observed in water controls. Even though the strongest signals were observed in abdomen of DSS treated mice with ProSense 680 probe and Cat K 680 FAST probes, we did not observe intense and localized activation signal in the region of the colon of DSS treated mice. The non specific signals observed in both diseased animals and water control animals could be generated by various sources including fecal contents, activation by skin or due to probe being metabolized or concentrated in liver and bladder. Software available with FMT2500 does not currently permit selection of ROI to specifically image the colon in vivo and so the background reflects the entire abdominal region.
Discussion
This study tested a series of cathepsin or MMP activatable NIRF probes for utility in ex vivo or in vivo detection and quantification of inflammation in two widely used murine models, DSS-induced acute injury in WT mice and chronic colitis in mice with targeted disruption of both IL-10 alleles. Ex vivo imaging demonstrated that all probes tested yielded strong signals in distal colon of DSS treated mice and cecum and colon of IL-10 null mice with low-level activation in other non-inflamed regions of intestine or in non-diseased controls. Strong and significant correlations between quantitative data for probe activation based on FRI ex vivo imaging in colon and colitis scores obtained from histologic sections suggest utility of ex vivo imaging with these probes for quantification of inflammation in both acute injury/inflammation and chronic colitis models. Some of these probes showed promise for in vivo imaging to detect colonic inflammation, but there are still some challenges to be overcome, particularly non-specific background and ability to validly localize signal to the colon.
The ProSense 680 probe was originally developed based on gene microarray data, which demonstrated that cathepsin B is dramatically up-regulated in lung cancer (29). Our prior studies and other published studies demonstrated that this probe is useful for ex vivo detection of intestinal adenomas in the Apc Min+ model (22, 23) and gastric tumors in a SMAD4+/− mice (30). Cathepsin activatable NIRF probes have been used successfully to detect inflammation in vivo and ex vivo in preclinical models of asthma (31), arthritis (8) and atherosclerosis (21) and one study used a different cathepsin-activatable probe in the piroxicam-activated IL-10 null colitis model (35). MMP-activatable probes have been used to detect tumors in azoxymethane (AOM) and AOM-DSS models of spontaneous and colitis-associated tumors (32). To our knowledge however, MMP probes have not been tested previously in acute inflammation or colitis models. Our data demonstrated that both cathepsin-based and MMP-based probes successfully detected colonic inflammation in DSS and IL-10 null models and therefore could potentially be used in preclinical studies monitoring incidence, location or severity of disease.
Use of activatable NIRF probes for detection of colitis ex vivo in mouse models of acute injury induced by DSS and chronic colitis due to IL-10 gene disruption
We first tested cathepsin- or MMP-activatable probes in the DSS model of acute colitis, which is mediated by DSS-induced injury to the colon epithelium and acute macrophage and neutrophil-mediated inflammation. Although not a model of T cell-mediated chronic inflammation, the DSS model is widely used to assess mediators of acute inflammation and interventions that may impact severity of DSS-induced injury.
Ex vivo, all cathepsin- and MMP-based probes, yielded signals in colon of DSS-treated mice that were significantly higher than water controls. Thus both cathepsin- or MMP-activated probes appear useful for detecting acute inflammation. FAST probes also showed promise but signals were not as strong, possibly because these probes were designed to be quickly biodistributed and metabolized/excreted. It is possible that increasing probe dose could enhance signal with FAST probe types. Ex vivo all probes yielded strong signals but the 680 series probes had the advantage of stronger signal and signal preservation after fixation. This could be particularly useful for assays of colitis severity in experiments including large number of animals.
Cathepsin B is a cysteine protease produced by macrophages and neutrophils that plays a role in promoting colitis in human IBD or DSS induced colitis mouse models (9, 18). Significant overlap between NIR fluorescence signal observed with ProSense 680 in sections of inflamed colon from DSS treated mice and cathepsin B immunofluorescence support the specificity of ProSense 680 in the detection of DSS-induced colitis.
The MMP-based probe MMPSense 680 and cathepsin K based probe Cat K 680 FAST were tested in IL-10 null mice, a model of chronic, T cell inflammation with features in common with human IBD. Findings that ex vivo imaging detects probe activation in regions known to exhibit chronic colitis in the IL-10 null model further support the potential utility of use of these probes to monitor and quantify colitis in preclinical IBD models.
The signal intensity of the activated probes correlates with histologic colitis scores
Conventional histology enabled confirmation of colitis disease activity in DSS or IL-10 null models versus controls. Data from both DSS induced acute colitis model and IL-10 null chronic colitis model suggested that the quantification of activated NIRF probe by FRI yielded signals that strongly and significantly correlated with severity of histologic colitis score within and across animals tested. These results support the potential utility of NIRF probes for use in studies assessing impact of an intervention on colitis severity.
ProSense 680 yields the highest signals in the DSS colitis model ex vivo or in vivo
In a prior study we reported data that ProSense 680 was activated to a much greater degree by a tumor in the IL-10 null mouse model than by colitis (23). Our current study suggests that ProSense 680 is significantly activated by acute and chronic colon inflammation. For clinical GI disease screening and diagnosis, there is a need for more specific probes to effectively distinguish inflammation from tumor because proteases are up-regulated in both colitis and tumors. Prior studies using microarray-based gene expression profiling on mouse colon tumors suggested that MMP-9 is a dramatically up-regulated gene (33). In the present study, although MMP-activated probes detected inflammation ex vivo in both DSS and IL-10 null models, the strongest signals in ex vivo imaging were observed with ProSense 680. Similarly, in vivo imaging in DSS treated animals revealed the strongest signals with ProSense 680 probe. Thus, both ex vivo and in vivo results indicate that ProSense 680 probe is the most effective of commercially available probes for detection of acute DSS-induced inflammation of colon. A prior study (34) using a different cathepsin activatable NIRF probe reported promising in vivo and ex vivo detection of colitis in the piroxicam-inducible IL-10 null model. In this study, significantly higher signals than background were obtained with FMT-in vivo imaging. Co-registration with MR localized signals to the colon. However, as found here, background fluorescence was observed in the colitis model outside of the region of the colon, which may reflect secretion of activated cathepsins (34). Background in non-diseased controls was somewhat lower in the prior report (34) than in the present study, and this may reflect probe-specific differences. Together, the prior and current study suggests that in vivo imaging of colitis with NIRF probes will still require some refinements for optimal application.
We found that feeding with liquid diet for 4 days reduced but did not eliminate non-specific abdominal fluorescence signals and was better than other pretreatments such as Trilyte or fasting. Liquid diet even for ex vivo imaging, reduced background fluorescence. This mild GI tract preparation was well tolerated by animals treated with DSS or IL-10 null mice with colitis. In probe injected control animals, the background signals detected in abdomen ROI during in vivo imaging likely derive from probe distribution and activation in liver, bladder, skin and joints. Compared with signals detected in water controls, consistently higher in vivo fluorescent signals, anatomically localized to the abdominal region, were observed in DSS treated animals injected with three different probes (ProSense 680, Cat K 680 FAST and MMPSense 680). However, in vivo signals were lowest with probes excited at the 750 nm wavelength, and their signal intensity was not higher than that in control animals. The probes that yielded the weakest signals during in vivo imaging were also the ones that yielded weaker signals ex vivo. More analyses will be needed to establish why particular probes are more sensitive for in vivo imaging. It is also note worthy that our in vivo analyses probably underestimate the increase in signal in DSS-treated animals as we used a region of interest encompassing entire abdomen in both water controls and DSS treated mice to ensure we fully detected background signal.
One strategy to improve in vivo imaging is to limit the ROI specifically to the colon. We have attempted this and obtained higher signals above background in DSS colitis versus water controls. However, specific location of ROI to the colon needs to be better validated by comparisons with other methods such as MRI and in multiple mice to account for inter-animal variability in colon location.
Potential advantages in clinic
There is increasing interest in use of molecular imaging to improve detection and diagnosis of IBD (35–37). The current data demonstrating ability to detect and quantify inflammation by imaging the surface of colon ex vivo indicates the potential that activatable NIRF probes might be combined with colonoscopy to improve disease detection. Recent studies have successfully used NIR endoscopy systems in preclinical cancer models to detect esophageal adenocarcinoma (38) and colonic adenomas (39, 40). The potential advantages of NIR combined with endoscopy include higher sensitivity or lower miss rates. NIR detection on medical endoscopes for clinical application has been pioneered for targeting flat and depressed colonic neoplasms in clinical imaging (41). Combination of probes with capsule endoscopy as we have tested experimentally in preclinical cancer models (23) could also offer improved ability to detect small bowel disease. However, there remain significant challenges to overcome before activatable NIRF probes might be applied in the clinic with conventional colonoscopy or capsule endoscopy. First, the probes must be injected and this will require FDA approval and clinical data regarding safety. Secondly, the existing cathepsin and MMP probes appear to be activated by both inflammation and precancerous/cancerous lesions. Ultimately it would be desirable to identify probes specific to inflammation vs. cancer or to different types of inflammation such as acute, infectious or chronic. Despite these limitations and challenges, the current findings provide evidence and proof of principle that activatable NIRF probes have utility for ex vivo and potentially in vivo detection and quantification of colonic inflammation in mouse models and may yield significant benefits in terms of time, labor and number of animals needed to monitor disease in existing or new rodent models of intestinal inflammation, and for testing of therapies.
Moreover, because one advantage of working in the optical imaging is the ability to image multiple fluorochromes, this technique can be expanded to allow the measurement of multiple molecular signals. In this study, we demonstrate that both cathepsin- and MMP-based probes alone or combined successfully detected colitis. Characterizing inflammation lesions for activation of multiple molecular mediators even in preclinical studies holds promise for better understanding of biologic pathways activated in lesions or response to therapies.
Supplementary Material
Acknowledgments
Source of Funding: DK047769, CA105417 and University Cancer Research Fund (UCRF)
We thank Jessica M. Bradford (former student in Department of Physiology, Morgan State University) for helping with animal handling. We acknowledge Kirk McNaughton and the Histology Core Facility of the Department of Cell Biology and Physiology at UNC-Chapel Hill and Dr. Hong Yuan from the Biomedical Research Imaging Center (BRIC) in UNC for invaluable assistance and input. Thanks also to Mr. Josh Robbs from the Department of Cell Biology and Physiology at the University of North Carolina at Chapel Hill who provided assistance in preparing this manuscript for submission.
Footnotes
Conflicts of Interest: no conflicts of interest.
References
- 1.Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. doi: 10.1007/s00535-010-0219-3. [DOI] [PubMed] [Google Scholar]
- 2.Vucelic B. Inflammatory bowel diseases: controversies in the use of diagnostic procedures. Dig Dis. 2009;27:269–277. doi: 10.1159/000228560. [DOI] [PubMed] [Google Scholar]
- 3.Efthymiou M, Taylor AC, Kamm MA. Cancer surveillance strategies in ulcerative colitis: the need for modernization. Inflamm Bowel Dis. 2011;17:1800–1813. doi: 10.1002/ibd.21540. [DOI] [PubMed] [Google Scholar]
- 4.Iacucci M, Panaccione R, Ghosh S. Advances in novel diagnostic endoscopic imaging techniques in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:873–880. doi: 10.1097/MIB.0b013e318280143f. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt C, Bielecki C, Felber J, et al. Surveillance strategies in inflammatory bowel disease. Minerva Gastroenterol Dietol. 2010;56:189–201. [PubMed] [Google Scholar]
- 6.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa M, Regino CA, Choyke PL, et al. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 9.Hausmann M, Obermeier F, Schreiter K, et al. Cathepsin D is up-regulated in inflammatory bowel disease macrophages. Clin Exp Immunol. 2004;136:157–167. doi: 10.1111/j.1365-2249.2004.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makitalo L, Kolho KL, Karikoski R, et al. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand J Gastroenterol. 2010;45:862–871. doi: 10.3109/00365520903583863. [DOI] [PubMed] [Google Scholar]
- 11.Martinesi M, Treves C, Bonanomi AG, et al. Down-regulation of adhesion molecules and matrix metalloproteinases by ZK 156979 in inflammatory bowel diseases. Clin Immunol. 2010;136:51–60. doi: 10.1016/j.clim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Santana A, Medina C, Paz-Cabrera MC, et al. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol. 2006;12:6464–6472. doi: 10.3748/wjg.v12.i40.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischbeck A, Leucht K, Frey-Wagner I, et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut. 2011;60:55–65. doi: 10.1136/gut.2009.201988. [DOI] [PubMed] [Google Scholar]
- 14.Lakatos G, Hritz I, Varga MZ, et al. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30:289–295. doi: 10.1159/000336995. [DOI] [PubMed] [Google Scholar]
- 15.Altadill A, Eiro N, Gonzalez LO, et al. Comparative analysis of the expression of metalloproteases and their inhibitors in resected crohn’s disease and complicated diverticular disease. Inflamm Bowel Dis. 2012;18:120–130. doi: 10.1002/ibd.21682. [DOI] [PubMed] [Google Scholar]
- 16.Kirkegaard T, Hansen A, Bruun E, et al. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut. 2004;53:701–709. doi: 10.1136/gut.2003.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedica F, Pecori S, Vergine M, et al. Cathepsin-k as a diagnostic marker in the identification of micro-granulomas in Crohn’s disease. Pathologica. 2009;101:109–111. [PubMed] [Google Scholar]
- 18.Menzel K, Hausmann M, Obermeier F, et al. Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin Exp Immunol. 2006;146:169–180. doi: 10.1111/j.1365-2249.2006.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 20.Korideck H, Peterson JD. Noninvasive quantitative tomography of the therapeutic response to dexamethasone in ovalbumin-induced murine asthma. J Pharmacol Exp Ther. 2009;329:882–889. doi: 10.1124/jpet.108.147579. [DOI] [PubMed] [Google Scholar]
- 21.Jaffer FA, Vinegoni C, John MC, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002;122:406–414. doi: 10.1053/gast.2002.30990. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Morgan D, Cecil G, et al. Biochromoendoscopy: molecular imaging with capsule endoscopy for detection of adenomas of the GI tract. Gastrointestinal endoscopy. 2008;68:520–527. doi: 10.1016/j.gie.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KL, Fuller CR, Dieleman LA, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 25.Yoon SM, Myung SJ, Kim IW, et al. Application of near-infrared fluorescence imaging using a polymeric nanoparticle-based probe for the diagnosis and therapeutic monitoring of colon cancer. Digestive diseases and sciences. 2011;56:3005–3013. doi: 10.1007/s10620-011-1685-z. [DOI] [PubMed] [Google Scholar]
- 26.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. The Journal of clinical investigation. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirtz S, Neurath MF. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int J Colorectal Dis. 2000;15:144–160. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 28.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 29.Cordes C, Bartling B, Simm A, et al. Simultaneous expression of Cathepsins B and K in pulmonary adenocarcinomas and squamous cell carcinomas predicts poor recurrence-free and overall survival. Lung Cancer. 2009;64:79–85. doi: 10.1016/j.lungcan.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Ding S, Eric Blue R, Chen Y, et al. Molecular imaging of gastric neoplasia with near-infrared fluorescent activatable probes. Molecular imaging. 2012;11:507–515. [PMC free article] [PubMed] [Google Scholar]
- 31.Kossodo S, Pickarski M, Lin SA, et al. Dual in vivo quantification of integrin-targeted and protease-activated agents in cancer using fluorescence molecular tomography (FMT) Mol Imaging Biol. 2010;12:488–499. doi: 10.1007/s11307-009-0279-z. [DOI] [PubMed] [Google Scholar]
- 32.Yoon SM, Myung SJ, Ye BD, et al. Near-infrared fluorescence imaging using a protease-specific probe for the detection of colon tumors. Gut and liver. 2010;4:488–497. doi: 10.5009/gnl.2010.4.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser S, Park YK, Franklin JL, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cattaruzza F, Lyo V, Jones E, et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology. 2011;141:1864–1874. e1861–1863. doi: 10.1053/j.gastro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Machtaler S, Bettinger T, et al. Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin-targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology. 2013;267:818–829. doi: 10.1148/radiol.13122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshpande N, Lutz AM, Ren Y, et al. Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology. 2012;262:172–180. doi: 10.1148/radiol.11110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBride HJ. Nuclear imaging of autoimmunity: focus on IBD and RA. Autoimmunity. 2010;43:539–549. doi: 10.3109/08916931003674766. [DOI] [PubMed] [Google Scholar]
- 38.Habibollahi P, Figueiredo JL, Heidari P, et al. Optical Imaging with a Cathepsin B Activated Probe for the Enhanced Detection of Esophageal Adenocarcinoma by Dual Channel Fluorescent Upper GI Endoscopy. Theranostics. 2012;2:227–234. doi: 10.7150/thno.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Miller SJ, Joshi BP, et al. In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut. 2013;62:395–403. doi: 10.1136/gutjnl-2011-301913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gounaris E, Martin J, Ishihara Y, et al. Fluorescence endoscopy of cathepsin activity discriminates dysplasia from colitis. Inflammatory bowel diseases. 2013;19:1339–1345. doi: 10.1097/MIB.0b013e318281f3f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TD. Targeted imaging of flat and depressed colonic neoplasms. Gastrointestinal endoscopy clinics of North America. 2010;20:579–583. doi: 10.1016/j.giec.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.