Figure 1.
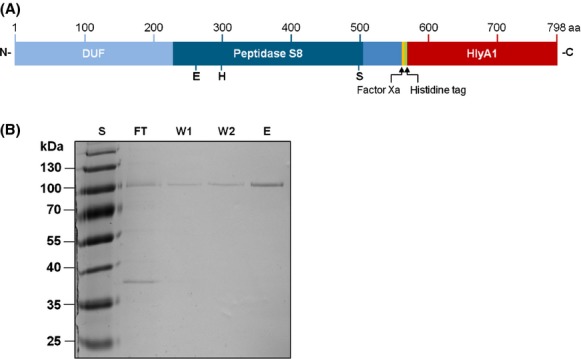
Expression in Escherichia coli and purification of SprP. (A) Schematic representation of the SprP-HlyA1 fusion protein used for expression and secretion in E. coli via the type I secretion system; SprP (blue) is shown with the DUF, Peptidase S8 domain, and the short C-terminal extension. Putative active site residues located within the Peptidase S8 domain are indicated. HlyA1 secretion signal (red) was fused to the C-terminus of SprP. Additionally, a six residue histidine tag (green) and a factor Xa recognition site for cleavage of the secretion signal (yellow) were inserted. (B) Analysis of SprP-HlyA1 fusion protein isolated from E. coli culture supernatants. Ten microliter each of different fractions obtained after chromatography on a Ni-NTA column were analyzed by SDS-PAGE and subsequent staining with Coomassie Brilliant Blue. The theoretical Mr of the SprP fusion protein is 88 kDa. Lanes show fractions of FT, first (W1) and second (W2) washing step with Tris-HCl buffer containing 20 and 30 mmol/L imidazole, respectively, and final elution with Tris-HCl buffer containing 250 mmol/L imidazole (E). S = Mr standard proteins (PageRuler Plus Prestained Protein Ladder, Fermentas, Sankt Leon-Rot Germany), aa = numbering of amino acids. DUF, domain of unknown function; SDS-PAGE, sodiumdodecyl sulfate polyacrylamide gel electrophoresis; FT, flow through.
